Abstract
Background:
Understanding how frailty affects patients listed for transplantation has been identified as a priority research need. Frailty may be associated with a high risk of death or wait-list withdrawal, but this has not been evaluated in a large multicenter cohort of Canadian wait-listed patients.
Objective:
The primary objective is to evaluate whether frailty is associated with death or permanent withdrawal from the transplant wait list. Secondary objectives include assessing whether frailty is associated with hospitalization, quality of life, and the probability of being accepted to the wait list.
Design:
Prospective cohort study.
Setting:
Seven sites with established renal transplant programs that evaluate patients for the kidney transplant wait list.
Patients:
Individuals who are being considered for the kidney transplant wait list.
Measurements:
We will assess frailty using the Fried Phenotype, a frailty index, the Short Physical Performance Battery, and the Clinical Frailty Scale at the time of listing for transplantation. We will also assess frailty at the time of referral to the wait list and annually after listing in a subgroup of patients.
Methods:
The primary outcome of the composite of time to death or permanent wait-list withdrawal will be compared between patients who are frail and those who are not frail and will account for the competing risks of deceased and live donor transplantation. Secondary outcomes will include number of hospitalizations and length of stay, and in a subset, changes in frailty severity over time, change in quality of life, and the probability of being listed. Recruitment of 1165 patients will provide >80% power to identify a relative hazard of ≥1.7 comparing patients who are frail to those who are not frail for the primary outcome (2-sided α = .05), whereas a more conservative recruitment target of 624 patients will provide >80% power to identify a relative hazard of ≥2.0.
Results:
Through December 2019, 665 assessments of frailty (inclusive of those for the primary outcome and all secondary outcomes including repeated measures) have been completed.
Limitations:
There may be variation across sites in the processes of referral and listing for transplantation that will require consideration in the analysis and results.
Conclusions:
This study will provide a detailed understanding of the association between frailty and outcomes for wait-listed patients. Understanding this association is necessary before routinely measuring frailty as part of the wait-list eligibility assessment and prior to ascertaining the need for interventions that may modify frailty.
Trial Registration:
Not applicable as this is a protocol for a prospective observational study.
Keywords: frailty, transplantation, transplant, cohort study, wait list, dialysis
Abrégé
Contexte:
La compréhension de l’incidence de la fragilité sur les patients en attente d’une greffe rénale a été désignée comme un besoin prioritaire de recherche. La fragilité pourrait être associée à un risque élevé de mortalité ou de se voir retiré de la liste d’attente pour une transplantation, mais elle n’a jamais été évaluée dans une vaste cohorte multicentrique de patients canadiens en attente d’une greffe.
Objectifs:
Le principal objectif consiste à déterminer si la fragilité d’un patient l’expose à un plus grand risque de décès ou de retrait permanent de la liste d’attente pour une greffe. Nous souhaitons également vérifier s’il existe un lien entre la fragilité et le nombre d’hospitalisations, la qualité de vie et la probabilité d’être accepté sur la liste d’attente.
Type d’étude:
Étude de cohorte prospective.
Cadre:
Sept sites disposant d’un programme de transplantation rénale évaluant les patients en vue de leur inscription sur la liste d’attente pour une greffe.
Sujets:
Des candidats à la liste d’attente pour une transplantation rénale.
Mesures:
La fragilité sera évaluée à l’aide du Phénotype de Fried (un indice de la fragilité), du test SPPB (Short Physical Performance Battery) et de l’échelle Clinical Frailty Scale au moment de l’inscription sur la liste d’attente pour une transplantation. Nous mesurerons la fragilité des patients de leur orientation vers le programme jusqu’à leur inscription sur la liste, puis sur une base annuelle après leur inclusion dans un sous-groupe de patients.
Méthodologie:
Le résultat principal, soit un composite du délai avant le décès ou le retrait permanent de la liste, sera comparé entre les patients fragiles et non fragiles, et tiendra compte des risques concurrents découlant de la transplantation selon que l’organe provient d’un donneur vivant ou décédé. Les résultats secondaires comprendront le nombre d’hospitalisations et leur durée, les variations de la fragilité et de la qualité de vie au fil du temps (pour un sous-groupe de patients), de même que les probabilités d’être inscrit sur la liste d’attente. Le recrutement de 1 165 patients nous permettrait d’obtenir un risque relatif d’au moins 1,7 dans plus de 80 % des cas lors de la comparaison des patients fragiles à ceux qui ne le sont pas pour le résultat principal (double erreur alpha = 0,05), alors que ce risque relatif serait de 2,0 avec un objectif de recrutement plus conservateur de 624 patients.
Résultats:
Un total de 665 évaluations de la fragilité (tant pour le résultat primaire que pour les résultats secondaires, y compris les mesures répétitives) a été complété en décembre 2019.
Limites:
Les résultats et leur analyse devront tenir compte des possibles variations entre les différents sites en ce qui concerne les processus d’aiguillage et d’inscription sur les listes d’attente pour une greffe.
Conclusion:
Cette étude fournira une compréhension détaillée de l’association entre la fragilité et les résultats cliniques pour les patients en attente d’une greffe. La compréhension de cette association est nécessaire avant d’inclure systématiquement la mesure de la fragilité au processus d’évaluation de l’admissibilité à la liste d’attente et avant d’établir le besoin de procéder à des interventions susceptibles de modifier la fragilité du patient.
Introduction
Background
Patients who are wait listed for a kidney transplant should have a good probability of surviving beyond waiting times
It is well established that kidney transplantation offers improved survival over dialysis.1 In 2017, more than 3000 Canadians were waiting for a kidney transplant, nearly 3 times more than those who actually received one.2 As this difference continues to grow, wait-list guidelines emphasize that kidney transplantation should be reserved for those who will benefit,3,4 and transplant candidates should have a good probability of surviving beyond waiting times.4-6 Assessing patients for the wait list involves a detailed assessment by transplant specialists to identify contraindications to transplantation. Patients without contraindications who are subsequently activated to the list require periodic testing to determine the need for wait-list removal if they develop new contraindications. Contraindications are factors that would markedly reduce the probability of survival on the wait list or patient/organ survival soon after transplantation.3,4,7,8 We previously identified that these established contraindications4 (ie, dementia, active malignancy, and multisystem disease) are typically associated with a ≤50% chance of surviving to transplantation (based on a national median wait time of ≈4 years).5 Furthermore, we showed that wait-listed patients with a high baseline mortality risk are more likely to be harmed from transplantation.6 Although patients who are active on the list are generally healthier than non-wait-listed or inactive dialysis patients,9,10 they are still at risk of death11 or of developing a permanent contraindication requiring them to be withdrawn from the list.9
A large proportion of patients who are wait listed will never receive a kidney transplant because of death or withdrawal from the wait list
In the United States in 2015, >9000 patients either died or were removed from the wait list due to deteriorating health.12 In another study from the United States, ≈50% of elderly wait-listed patients (>60 years) died prior to deceased donor transplantation,13 In Canada, ≈300 patients (10%) died or were withdrawn from the wait list in 2017 alone.2 These patients would not have had a contraindication at the time of listing but may have been at an unrecognized risk of poor health outcomes. One recent analysis used a risk prediction tool to determine the likelihood of outcomes for transplant candidates including withdrawal, death, or transplantation.14 While the model had good discrimination, the study was conducted in the United States where transplantation practice and the probability of wait-list survival differ compared with Canada. This study suggested that “granular-level data such as frailty status14” is needed to improve risk prediction for wait-listed patients.
Wait listing patients with little probability of receiving a kidney transplant has negative consequences
As identified by a study conducted in the United States, the direct patient costs associated with a wait-list evaluation can exceed US$1000.15 The societal costs of wait listing patients with little chance of receiving a transplant may also be high. The time required to determine wait-list eligibility (which is >6 months in some cases)16 and the need for multiple diagnostic tests16 are limited resources that are needed for all wait-list candidates. Committing these resources to patients who are unlikely to receive a transplant may delay the work-up for those with a higher chance of success. Most importantly, wait listing patients with little chance of receiving a transplant may be associated with direct patient harm. In a large thematic synthesis of 22 qualitative studies representing 795 patients, a common theme that was observed was that patients perceive the work-up and testing for the kidney transplant wait list to be burdensome.17 Furthermore, uncertainty about eligibility, the demands of being worked-up, and waiting times that exceed expectations resulted in patient anxiety, concern of inequality, disillusionment, and despair.17
Frailty is associated with poor health outcomes among dialysis patients and kidney transplant recipients
Accumulating deficits across many domains including health, mobility, function, and cognition puts an individual at a higher risk for poor health outcomes and is often referred to as “frailty.”18 The 2 most widely used and validated methods to evaluate frailty are the Fried Frailty Phenotype Assessment (Fried Phenotype)19,20 and a frailty index (FI) approach.21,22 Irrespective of which tool is used, frailty is associated with an increased risk for mortality and hospitalization and a deterioration in quality of life after dialysis initiation.23-26 Similarly, after adjustment for comorbidity, demographics, and other recipient factors, patients who are frail are at a higher risk of multiple poor health outcomes after transplantation including mortality, hospitalization, and reduced quality of life.27-32
Despite the association between frailty and reduced health outcomes, an evaluation of frailty is not a standard component of the kidney transplant wait-list eligibility assessment
Most studies of the effect of frailty on outcomes are based on assessments at the time of transplantation.27-30 This is a late and impractical time to guide decisions around transplantation. A frailty assessment at the time of referral may help to identify patients who are unlikely to complete the work-up.31,33 However, wait-list referral is not a defined time-point and may vary across centers and individual nephrologists. Furthermore, the time between referral and activation may be lengthy,16 and a patients’ frailty status may change over this time.31,33 Therefore, evaluating outcomes after listing based solely on a frailty assessment at referral may be subject to bias. In contrast, patients are activated to the wait list once their testing and work-up are complete. Therefore, this is the most defined and practical time-point to evaluate the impact of frailty on wait-list outcomes. One recent single-center United States study evaluated frailty status (using the Fried Phenotype) and mortality among patients being evaluated for a kidney transplant.31 Importantly, 24% of patients who were frail were less likely to be listed,31 and frailty was associated with a 2-fold increase in the risk of death (but did not improve risk prediction). While informative, findings in this study may not be generalizable to Canadian patients, for whom outcomes on dialysis and after kidney transplant differ.34-36 Furthermore, this study did not evaluate other outcomes (probability of wait-list withdrawal) or other frailty assessment tools. While comorbidities (that are more common with advancing age) are the main method used to ascertain wait-list eligibility, some patients who are frail may have a small burden of comorbidity and neither age nor comorbidity modify the association between frailty and poor health outcomes.23,28,37,38 This suggests that patients who are frail without a comorbidity contraindication might be deemed eligible and listed, but unlikely to receive a kidney transplant.
Why is this study important for patients and providers?
This study aims to fill a knowledge gap that is globally recognized.39 Physicians commonly perceive reduced “functional status” (a component of frailty) as a characteristic of a suboptimal wait-list candidate,40 but nephrologist perceived frailty has little agreement with measured frailty.41 Kidney organs are a scarce resource and guidelines have identified that transplant candidates should have a good probability of surviving beyond waiting times.4-6 If this study finds that patients who are frail are unlikely to receive a transplant because of death or withdrawal, ascertaining frailty status may become standard of care and identification of this vulnerable patient group will help to identify those that may benefit from future interventions that may help to mitigate frailty. Without a clear understanding of the impact of frailty on outcomes and how frailty changes over time, it is unclear whether future interventions are even necessary. Discussing frailty status and anticipated outcomes would be a crucial part of the informed decision-making process for potential wait-list candidates. Patients on the wait list who are identified as frail may choose to forgo the burden of testing and distress of uncertainty as to their wait-list status17 in the face of little chance of receiving a transplant. Finally, providers could use an annual frailty assessment to reevaluate and inform patients who are already listed. In contrast, if frailty is not associated with poor health outcomes, it would emphasize that patients without other contraindications should not be denied the opportunity to be wait listed based on measured or perceived40 frailty.
Objectives
In a cohort of patients accepted to the kidney transplant wait list:
Primary: To determine whether patients who are frail are at a higher risk of death or permanent withdrawal from the wait list compared with nonfrail patients.
Secondary: (1) To determine whether frailty improves risk prediction when added to an existing model for mortality/wait-list withdrawal. (2) To assess changes in frailty and frailty severity among wait-listed patients and candidates. (3) To identify whether patients who are frail are at a higher risk of hospitalization compared with nonfrail patients. (4) To identify whether patients who are frail have a lower quality of life compared with nonfrail patients. (5) To compare the level of agreement between objective and subjective frailty assessments. (6) In a subset of patients who are referred for the wait list: to determine whether patients who are frail are less likely to be accepted to the list compared with nonfrail patients.
Methods
Study Design and Population
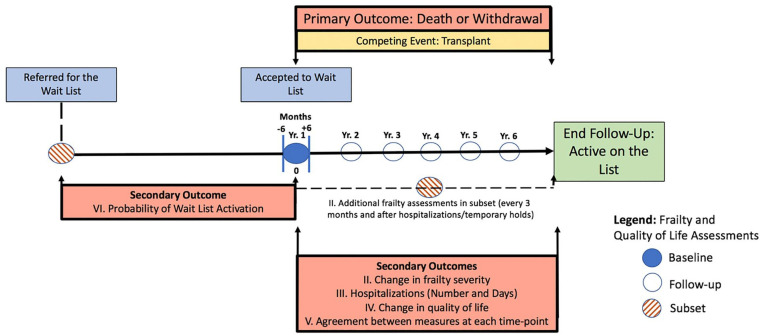
This will be a prospective cohort study of Canadian adult patients from 7 sites with established renal transplant programs that evaluate patient eligibility for the kidney transplant wait list. Patients will be recruited over 5 years and followed for an additional year after recruitment is complete (Figure 1). Patients will be excluded if they are unwilling to consent or unable to complete the frailty measures (without a substitute decision maker).
Figure 1.
Patient flow diagram for each objective.
Exposure Assessment
Frailty will be measured using the Fried Phenotype19 (primary tool), and the following secondary tools: an FI,21 the Short Physical Performance Battery (SPPB),42 and the Clinical Frailty Scale (CFS).37,43,44
Patients from peripheral centers affiliated with participating sites in London, Montréal, Hamilton, Winnipeg, and Regina are assessed in-person by a nephrologist who determines acceptance to the wait list. They have prebooked appointments and are identified directly by the site-lead as candidates for study inclusion. Site research coordinators will conduct frailty assessments on the day of this evaluation. For Halifax and St. John, acceptance to the list is made by a committee in Halifax after the local work-up is complete, inclusive of an in-person assessment by the patient’s primary nephrologist. Eligible patients will be identified by the Nova Scotia Health Authority (NSHA) transplant recipient coordinators. Local study coordinators will perform frailty assessments close to the wait-list committee review date. It is possible that patients at any participating site will be evaluated for the wait list but require additional testing before acceptance. When feasible, if this period exceeds 6 months, the assessment will be repeated. It is also possible that eligible patients will be accepted to the wait list without completing their frailty assessments. Therefore, frailty assessments after acceptance (using a target of within 6 months) will also be accepted as the baseline measure. This practical time frame will avoid needless patient exclusion because of any difficulties in timing the frailty assessments to directly coincide with the wait-list activation date.
Additional frailty assessments (for secondary objectives) will be performed when feasible, and it is anticipated that these assessments will only be available in a small subset of patients. These include an additional frailty assessment during the time of wait-list referral (prior to activation) for secondary objective VI and follow-up assessments annually ±3 months for patients directly followed by any participating site for secondary objective II. Acknowledging the potentially dynamic nature of frailty,45 when feasible, additional frailty assessments will also be performed every 3 months and after any hospitalization events (which can worsen frailty severity)46 or temporary holds for patients at the primary site.
Frailty Measures
The Fried Phenotype (Table 1)19 is a valid measure of frailty that classifies patients as frail if they have 3 of 5 of unintentional weight loss, exhaustion, weakness, slowness, and low activity. This measure was chosen as the primary assessment tool due to the breadth of prior study,23,24,38 validity, and to permit comparisons to the only known study that has evaluated frailty for a cohort of wait-listed patients.31
Table 1.
Fried Phenotype: Frail: ≥3 Factors Present.
| 1. Unintentional Weight Loss:>10 pounds unintentional weight loss in prior year |
| 2. Exhaustion: Answers “3-4 days or most of the time to the following: How often in the last week did you feel that everything was an effort or you could not get going?” |
| 3. Muscle Weakness: Grip strength: lowest 20% by gender,
height Men: Threshold based on body mass index: ≤24: ≤29 kg, 24.1-28: ≤30 kg, >28: ≤32 kg Women: ≤23: ≤17 kg, 23.1-26: ≤17.3 kg 26.1-29: ≤18 kg, >29: ≤21 kg |
| 4. Slowness: Walking time/15 feet: slowest 20% by gender,
height Men: height ≤173 cm≥7 seconds, height >173 cm≥6 seconds Women: height ≤159 cm≥7 seconds, height >159 cm≥6 seconds |
| 5. Low Levels of Activity: Kcals/week: lowest 20% (males
<383/week, females <270/week) Based on short version of the Minnesota Leisure Time Activity questionnaire. Activities: walking, chores, mowing, raking, gardening, hiking, jogging, biking, dancing, aerobics, bowling, golf, tennis, racquetball, calisthenics, swimming. Kcals/week calculated with a standardized algorithm |
The SPPB (Supplemental File 1)42 is a tool that is used to measure physical function based on the completion of a timed walk, tests of standing balance, and a timed series of 5 attempts to stand from a chair with crossed arms.42 It is continuous and scored from 0 (lowest degree of lower extremity functioning) to 12. It is associated with mortality and hospitalization and can be compared across populations. It has been evaluated in dialysis patients47-49 among older patients referred for the transplant wait list50 and in patients prior to transplantation.51,52 An FI is a measure of deficit accumulation with characteristic properties,21 that is, cohort-specific and contains 30 or more variables across multiple domains. The FI score is the ratio of deficits present in an individual/the total number of index deficits with scores ranging from 0 to 1. An FI approach has been validated in many populations, regardless of the items used,21,53-56 and FIs can use health record data and patient self-report items.53 We developed a transplant wait list–specific FI (Table 2) using a standardized approach55 with expert panel input from a diverse group of stakeholders (a geriatrician, 3 transplant nephrologists, and a general nephrologist) for content validity. We tested this index in a cross section of transplant candidates from Halifax. Index variables were present in at least 2 patients in the study. The mean score was 0.15 ± 0.10 (5 items) and the maximum score was 0.44 (16 items) (unpublished data). The index properties were as expected for a chronic disease population21 (normally distributed, peak score <0.70). The CFS (Supplemental File 2) is an overall clinician gestalt of frailty using a rating scale scored from 1 (very fit) to 8 (very severely frail). The CFS is has high inter-rater reliable (intraclass correlation coefficient of 0.97)44 and is highly and moderately correlated with the FI in the general population (r = .80)44 and dialysis population (r = .57),43 respectively. A higher frailty severity using the CFS is associated with mortality in the dialysis population (inclusive of kidney transplant wait-list candidates).37 In this study, the CFS score will be assessed for each wait-list candidate by a physician with clinical knowledge of the patient or the wait-list eligibility assessor.
Table 2.
Kidney Transplant Wait-List FI.
| Social/cognitive (6 items) | Functional (9 items) | Mobility (10 items) | Comorbidity (12) |
|---|---|---|---|
| • Socializes rarely • Emotional problems interfere with activities • Cut down on work because of emotional problems • Feeling lonely • Abnormal word recall • Impaired clock draw |
• Needs help with: • Meals, Shopping • Chores, Finances • Impaired in • Carrying groceries • Carry 10 pounds • Weak grip strength • Cut down work due to physical health • Cut down activity (last month) |
• Ltd in bending, kneeling, stooping • Help up/down stairs • Help in/out of chair • Ltd for one flight of stairs • Limited walking 100 m • Requires a cane/walker • Low physical activity • Slow walking speed • Exhaustion: self-report • Infrequent walking |
• Congestive heart failure, coronary artery disease,
cerebrovascular disease • Chronic lung disease, diabetes, musculoskeletal disease • Prior malignancy • Polypharmacya • Weight loss • Low albuminb • Self-rated low health • Self-rated worsening of health (last year) |
>10 meds/d.
<30 g/L.
The Fried Phenotype avoids potential bias from clinician impression19 and has been the most extensively used frailty assessment tool in dialysis and transplantation.23,24,27-31,38 It is easy to interpret, has been validated in multiple populations (including dialysis patients),20,57,58 and can be readily implemented into clinical practice. The SPPB is an objective measure that has been used to measure functional impairment in studies of dialysis patients.47-49 It avoids excess questionnaire burden and the activities are easy to perform serially.48,49 Properties of the SPPB have been evaluated in an elderly cohort of kidney transplant candidates.50 The FI includes additional information beyond function to evaluate frailty status, and small changes in frailty severity can be captured. Many of the individual items are routinely collected for potential wait-list candidates, making it a tool that can be implemented into clinical care. Some studies suggest using the Fried Phenotype and FI tools together.59 The CFS while subjective is the easiest to measure and provides an opportunity to assess how objective frailty measures compare with clinical gestalt. If risk prediction is improved with the objective tools, it will further emphasize the importance of incorporating these objectives measures into the wait-list assessment process. The prevalence of frailty varies depending on which tool is used and outcomes may differ. Use of all 4 tools will help identify which tool is most applicable to this population.
The primary coordinator in Halifax has trained site coordinators from active sites over teleconference prior to initiation on how to administer each tool. She is familiar with study procedures from the prior cross-sectional study and procedures have been detailed for each site using a standard operating procedures manual. Assessments involve physical examination, chart review, and questionnaires. This information will be collected using case report forms (CRFs) and entered into an online database by site coordinators.
Data Collection
Baseline data are routinely collected as part of the transplant wait-list eligibility assessment process and collated in charts provided to the assessor. Data will be collected prospectively on patients at each site by research coordinators. Specific demographic data will include the following: age at listing date, race, sex, employment status, weight, and height (for body mass index). Comorbid conditions (based on chart documentation) will include diabetes (type I or type II), coronary artery disease (history of prior myocardial infarction, coronary angioplasty, or on angiography), congestive heart failure (or echocardiographic evidence of systolic or diastolic dysfunction), peripheral vascular disease (established with imaging studies, or prior intervention), cerebrovascular disease (stroke or transient ischemic attack), history of prior malignancy (including type), chronic obstructive lung disease, hypertension, liver disease, prior failed kidney transplant, bone/joint disease, cause of end-stage kidney disease, and history of depression. Dialysis characteristics will include receiving dialysis (yes/no), modality (peritoneal dialysis, in-center, intensive hemodialysis), dialysis access (catheter, fistula, graft), dialysis vintage, and time from referral to acceptance. Baseline laboratory data will include the following: serum albumin, blood type, human leukocyte antigen, and peak panel reactive antibody (PRA) level.
Paper CRFs to assist with point-of-care data capture and to resolve data queries will be held at each center according to local regulations. The CRFs have been translated to French by the local study team for French-speaking participants in Montréal. Data will be sent to the primary site for entry into a computer database (REDCap). Data entered online will have a unique identifier but no identifying data.
Outcomes
The primary outcome (and outcome for secondary objective 1) will be composite of time to death or permanent wait-list withdrawal starting from the date of wait-list activation. Deaths for both active and inactive patients (ie, those on temporary hold) will be included. Permanent withdrawal will be defined as removal from the wait list without anticipated reactivation (acknowledging that even lengthy temporary holds may be reactivated),60 but in a sensitivity analysis, temporary holds of >6 months will be included as an event. Causes of death and/or withdrawal will be collected for each patient. Secondary outcomes will include (2) changes in frailty severity/proportion classified as frail; (3) number, cause, and duration of hospitalizations while on the wait list; (4) change in quality of life (using the EQ-5D which has been studied in wait-listed kidney transplant candidates)61; (5) agreement between measures; (6) probability of being listed. While an extensive assessment of posttransplant outcomes is not the goal of this study; if feasible, first-year outcomes after transplantation (death, graft failure) will be described for all enrolled patients.
Mitigation of Risk
Use of a prospective design will avoid misclassification bias from retrospective ascertainment of frailty status. Previously published transplant eligibility guidelines used Canadian expert opinion.4 However, acceptance may differ across participating centers affecting frailty prevalence. Capturing prevalence at each center will allow a better determination of the likelihood of this bias and it is less likely to affect outcomes associated with frailty as the tools are consistent. There is a risk of immortal time for all patients between the frailty assessment and acceptance to the list (or acceptance to assessment). When feasible, repeat assessments will be conducted to minimize this time to ±6 months. If the time period exceeds 6 months without a repeat assessment either before or after acceptance to the list, we will account for this using appropriate statistical techniques,62 if required. The impact of frailty on outcomes is unknown. Therefore, physicians assessing patients for the wait list and all site research coordinators will be blinded to the results of the 3 objective measures (each of which requires additional calculation), which will only be ascertained at the primary site after case report form data are uploaded to the web-based data entry system. Acknowledging that baseline data is required by physicians who evaluate patients for the wait list, it is anticipated that there will be a minimal amount of missing baseline characteristics. Similarly, outcomes are closely monitored for wait-listed patients. In the case of missing data, local centers will be recontacted to collect missing data from local information sources. Statistically, unresolved missing data will be managed using multiple imputation by chained equations prior to proposed analyses.63
Analysis
Count/percentages and means ± SD will be used to describe each frailty measure when appropriate. Histograms of FI, SPPB, and CFS scores will be displayed. The Fried Phenotype is a binary measure. The FI, SPPB, and CFS will be treated as continuous measures and all 3 tools will be analyzed as a nonlinear variable using splines. Descriptive statistics will be used to report baseline characteristics and outcomes for all patients.
Primary outcome
Similar to a previous study evaluating outcomes after wait listing,14 the effect of each frailty measure on death/permanent withdrawal will be analyzed using a competing risk approach with competing events of deceased and living donor transplantation, and a standard Cox survival analysis with censoring at transplantation in a secondary analysis. Each subcomponent of the primary outcome will also be evaluated using a similar approach. Adjusted time to death/permanent withdrawal will be analyzed using a proportional subdistribution hazards model (Fine and Gray approach).64 Proportionality will be tested using a validated approach for competing risk analyses65; nonproportional subdistribution hazards will be addressed using published methods.66 Variables for inclusion in the model will be those that are associated with a higher risk of death or withdrawal for wait-listed patients.14 The association between frailty and the death/withdrawal will be assessed across prespecified subgroups for effect measure modification. These include but are not limited to an age cutoff of ≥60 years,13 by sex, by blood type (O versus other), and by sensitization (PRA <80%, 80%-95%, >95%). In a sensitivity analysis, only the outcome of death/withdrawal due to deteriorating condition will be analyzed; withdrawal for other reasons will be treated as a competing event. In addition, frailty will be added to a previously developed wait-list survival model that evaluated the probability of deceased and living donor transplant and removal from the list due to death/deteriorating condition, or other reasons,14 and the predictive ability of adding frailty to this model will be quantified using reported methods for net reclassification improvement in survival analysis.67
Secondary outcomes
(2 and 4) Changes in frailty status, severity, and quality of life will be analyzed using generalized estimating equation approach to longitudinal modeling with logistic regression (for categorical measures) and generalized linear mixed effects modeling for continuous repeated measures. (3) Frequency, cause, and length of stay for each hospitalization event will be reported and differences in the risk for recurrent hospitalization comparing patients who are frail versus nonfrail patients will be reported using published statistical approaches.68 (5) Agreement will be assessed using appropriate approaches for continuous and categorical measures. (6) The time to listing for patients who are frail versus nonfrail patients who are referred will be reported and compared. Reasons for nonlisting will be described. For all comparisons, a P < .05 will be the threshold for statistical significance.
Sample Size Calculation and Threshold for Clinical Importance
We plan to enroll 1165 patients based on current recruitment. We showed that established wait-list contraindications are typically associated with a ≤50% chance of 4-year survival (the median national wait time), which is the threshold for clinical importance.5 The adjusted relative mortality hazard for frailty (using the Fried Phenotype) is >2 fold-higher in dialysis (hazard ratio [HR] = 2.24, 95% confidence interval [CI] = 1.60-3.15)24 and posttransplant (adjusted HR = 2.17, 95% CI = 1.01-4.65).28 In the only study of wait-listed patients, the adjusted HR was 2.19, 95% CI = 1.26-3.79). We assumed that 10% of the cohort would be frail (less than the 12% found in our preliminary analysis). Annual wait-list mortality is estimated at 3% to 5% for Canadian patients2,11,69 (lower than annual dialysis mortality of 17% in Canada).2 We assumed a total event rate (mortality and permanent withdrawal) of 10% per year (the rate in the latest Canadian Organ Replacement Register)2 and a nonoutcome attrition rate of 20% per year, including those who receive a transplant. Anticipated sample sizes corresponding to more conservative HRs of 1.7 to 2.0 for patients who are frail versus nonfrail patients are all below our expected recruitment. Furthermore, the probability of 4-year wait-list survival for patients who are frail is 50%, 47%, 45%, and 43%, using HRs of 1.7, 1.8, 1.9, and 2.0, respectively. Therefore, even with conservative HRs, our anticipated sample size will capture effect sizes that are above the threshold for clinical importance (≤50% 4-year wait-list survival). Required sample sizes for different relative hazards are noted in Table 3.
Table 3.
Required Sample Size for Different Relative Hazards (HRs).
| HR | Sample size (nonfrail/frail) | Annual survival rate (nonfrail/frail) | Four-year survival (nonfrail/frail) |
|---|---|---|---|
| 1.7 | 1147 (1032/115) | (0.90/0.84) | 0.66/0.50 |
| 1.8 | 910 (819/91) | (0.90/0.83) | 0.66/0.47 |
| 1.9 | 745 (670/75) | (0.90/0.82) | 0.66/0.45 |
| 2.0 | 624 (561/63) | (0.90/0.81) | 0.66/0.43 |
Note. HR = Hazard ratio.
The Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) guidelines will be used for all future publications resulting from this protocol.70
Results
The study has been approved at the lead site (NSHA, File #1020261) and all activated secondary sites. Recruitment has begun for 5 of 7 peripheral sites. Through December 2019, 665 assessments of frailty (inclusive of any study objective and repeated measures) using the CFS score, Fried Phenotype, and FI have been completed. Only a subset of individuals has completed their SPPB as this test was added at a later date.
Conclusions
This study will help inform the identification and management of patients who are frail at the time of assessment for eligibility for the kidney transplant wait list. Identifying the association between frailty and outcomes is necessary prior to undertaking interventions to modify frailty or support patients who are frail and who are being considered for the list. Importantly, this study (once externally validated) will also identify whether frailty adds further predictive value to an existing tool used to evaluate outcomes for wait-listed patients. This knowledge will support inclusion of a frailty assessment into transplant wait-list eligibility guidelines which internationally is a recognized knowledge gap.39 Finally, outcomes and health utility scores (using the EQ-5D) will inform a future cost-effectiveness analysis of wait-listing patients who are frail for transplantation.
Supplemental Material
Supplemental material, FrailtyProtocol2019Supplemental_Files for Frailty and the Kidney Transplant Wait List: Protocol for a Multicenter Prospective Study by Karthik K. Tennankore, Lakshman Gunaratnam, Rita S. Suri, Seychelle Yohanna, Michael Walsh, Navdeep Tangri, Bhanu Prasad, Nessa Gogan, Kenneth Rockwood, Steve Doucette, Laura Sills, Bryce Kiberd, Tammy Keough-Ryan, Kenneth West and Amanda Vinson in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors would like to thank the Canadian Nephrology Trials Network (CNTN) for feedback that was provided during the development of this protocol. The authors would like to thank Prosper Koto for his feedback regarding a future cost-effectiveness analysis and the Maritime SPOR SUPPORT Unit for feedback regarding and support of knowledge translation activities that are planned with this study.
Footnotes
Ethics Approval: The study has been approved at the lead site (Nova Scotia Health Authority REB#1020261) and each actively participating secondary site.
Consent for Publication: Not applicable.
Availability of Data and Materials: Not applicable.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors approved the submission of this manuscript for publication. A.V. has received funding from Paladin Labs Inc. for consulting services. K.W. has conducted consulting work for Paladin Labs Inc. N.T. has received grants and personal fees from AstraZeneca; personal fees from Otsuka, Janssen, Boehringer Ingelheim/Eli Lilly; and personal fees/other from Tricida Inc. K.K.T. has received funding from AstraZeneca, Janssen, Baxter, and Otsuka for consulting work, and investigator-initiated grant funding from Otsuka and Astellas.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has received funding support from the following funding sources: An Astellas investigator-initiated grant funding award (2016), a 2017 Kidney Foundation of Canada Biomedical Research Grant (currently known as the Kidney Health Research Grant), and a Canadian Institutes of Health Research Project Grant (2019).
ORCID iDs: Karthik K. Tennankore  https://orcid.org/0000-0002-7919-6709
https://orcid.org/0000-0002-7919-6709
Rita S. Suri  https://orcid.org/0000-0002-0519-3927
https://orcid.org/0000-0002-0519-3927
Bhanu Prasad  https://orcid.org/0000-0002-1139-4821
https://orcid.org/0000-0002-1139-4821
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2. Information CIfH. e-statistics on organ transplants, waiting lists and donors. https://www.cihi.ca/en/e-statistics-on-organ-transplants-waiting-lists-and-donors. Published 2016. Accessed February 2, 2018.
- 3. Batabyal P, Chapman JR, Wong G, et al. Clinical practice guidelines on wait-listing for kidney transplantation: consistent and equitable? Transplantation. 2012;94:703-713. doi: 10.1097/TP.0b013e3182637078. [DOI] [PubMed] [Google Scholar]
- 4. Knoll G, Cockfield S, Blydt-Hansen T, et al. Canadian society of transplantation: consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173:S1-S25. doi: 10.1503/cmaj.1041588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiberd BA, AlBugami MM, Panek R, Tennankore K. Contraindications to kidney transplantation: uneven grounds. Transplant Res. 2015;4:2. doi: 10.1186/s13737-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kiberd BA, Tennankore KK, West K. Eligibility for the kidney transplant wait list: a model for conceptualizing patient risk. Transplant Res. 2014;3:2. doi: 10.1186/2047-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta A, Montgomery RN, Bedros V, et al. Subclinical Cognitive impairment and listing for kidney transplantation. Clin J Am Soc Nephrol. 2019;14:567-575. doi: 10.2215/CJN.11010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McAdams-DeMarco MA, Daubresse M, Bae S, et al. Dementia, Alzheimer’s disease, and mortality after hemodialysis initiation. Clin J Am Soc Nephrol. 2018;13:1339-1347. doi: 10.2215/CJN.10150917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delmonico FL, McBride MA. Analysis of the wait list and deaths among candidates waiting for a kidney transplant. Transplantation. 2008;86:1678-1683. doi: 10.1097/TP.0b013e31818fe694. [DOI] [PubMed] [Google Scholar]
- 10. Gill JS, Tonelli M, Johnson N, Kiberd B, Landsberg D, Pereira BJ. The impact of waiting time and comorbid conditions on the survival benefit of kidney transplantation. Kidney Int. 2005;68(5):2345-2351. doi: 10.1111/j.1523-1755.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 11. Kim SJ, Fenton SS, Kappel J, et al. Organ donation and transplantation in Canada: insights from the Canadian Organ Replacement Register. Can J Kidney Health Dis. 2014;1:31. doi: 10.1186/s40697-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 annual data report: kidney. Am J Transplant. 2017;17(suppl 1):21-116. doi: 10.1111/ajt.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU. Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol. 2009;4(7):1239-1245. doi: 10.2215/CJN.01280209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hart A, Salkowski N, Snyder JJ, Israni AK, Kasiske BL. Beyond “median waiting time”: development and validation of a competing risk model to predict outcomes on the kidney transplant waiting list. Transplantation. 2016;100(7):1564-1570. doi: 10.1097/TP.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forbes RC, Rybacki DB, Johnson TB, et al. A cost comparison for telehealth utilization in the kidney transplant waitlist evaluation process. Transplantation. 2018;102:279-283. doi: 10.1097/TP.0000000000001903. [DOI] [PubMed] [Google Scholar]
- 16. Weng FL, Joffe MM, Feldman HI, Mange KC. Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis. 2005;46(4):734-745. doi: 10.1053/j.ajkd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 17. Tong A, Hanson CS, Chapman JR, et al. “Suspended in a paradox”—patient attitudes to wait-listing for kidney transplantation: systematic review and thematic synthesis of qualitative studies. Transpl Int. 2015;28(7):771-787. doi: 10.1111/tri.12575. [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-Manas L, Feart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 2013;68(1):62-67. doi: 10.1093/gerona/gls119-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156 [DOI] [PubMed] [Google Scholar]
- 20. Chowdhury R, Peel NM, Krosch M, et al. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr. 2017;68:135-142. doi: 10.1016/j.archger.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 21. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323-336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183:E487-E494. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bao Y, Dalrymple L, Chertow GM, et al. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071-1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 25. McAdams-DeMarco MA, Ying H, Olorundare I, et al. Frailty and health-related quality of life in end stage renal disease patients of all ages. J Frailty Aging. 2016;5(3):174-179. [PMC free article] [PubMed] [Google Scholar]
- 26. Kallenberg MH, Kleinveld HA, Dekker FW, et al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD—a systematic review. Clin J Am Soc Nephrol. 2016;11:1624-1639. doi: 10.2215/CJN.13611215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective cohort study. Ann Surg. 2017;266(6):1084-1090. doi: 10.1097/SLA.0000000000002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15:149-154. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091-2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99(4):805-810. doi: 10.1097/TP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McAdams-DeMarco MA, Olorundare I, et al. Frailty in patients being evaluated for kidney transplantation. American Transplant Congress (Abstract); 2015. https://atcmeetingabstracts.com/abstract/frailty-in-patients-being-evaluated-for-kidney-transplantation/. Accessed August 25, 2020.
- 32. McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and postkidney transplant health-related quality of life. Transplantation. 2018;102(2):291-299. doi: 10.1097/TP.0000000000001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, inflammatory markers, and waitlist mortality among patients with end-stage renal disease in a prospective cohort study. Transplantation. 2018;102(10):1740-1746. doi: 10.1097/TP.0000000000002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim SJ, Schaubel DE, Fenton SS, Leichtman AB, Port FK. Mortality after kidney transplantation: a comparison between the United States and Canada. Am J Transplant. 2006;6(1):109-114. doi: 10.1111/j.1600-6143.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 35. Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol. 2003;14(12):3270-3277. [DOI] [PubMed] [Google Scholar]
- 36. Goodkin DA, Young EW, Kurokawa K, Prütz KG, Levin NW. Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis. 2004;44(5 suppl 2):16-21. [DOI] [PubMed] [Google Scholar]
- 37. Alfaadhel TA, Soroka SD, Kiberd BA, et al. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol. 2015;10:832-840. doi: 10.2215/CJN.07760814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896-901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Segall L, Nistor I, Pascual J, et al. Criteria for and appropriateness of renal transplantation in elderly patients with end-stage renal disease: a literature review and position statement on behalf of the European Renal Association-European Dialysis and Transplant Association Descartes Working Group and European Renal Best Practice. Transplantation. 2016;100(10):e55-e65. doi: 10.1097/TP.0000000000001367. [DOI] [PubMed] [Google Scholar]
- 40. Ghahramani N, Sanati-Mehrizy A, Wang C. Perceptions of patient candidacy for kidney transplant in the United States: a qualitative study comparing rural and urban nephrologists. Exp Clin Transplant. 2014;12(1):9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salter ML, Gupta N, Massie AB, et al. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatr. 2015;15:52. doi: 10.1186/s12877-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94. [DOI] [PubMed] [Google Scholar]
- 43. Clark DA, Khan U, Kiberd BA, et al. Frailty in end-stage renal disease: comparing patient, caregiver, and clinician perspectives. BMC Nephrol. 2017;18:148. doi: 10.1186/s12882-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lanziotti Azevedo da Silva S, Campos Cavalcanti Maciel Ã, Maximo Pereira L, Domingues Dias JM, Guimarães de Assis M, Corrêa Dias R. Transition patterns of frailty syndrome in comunity-dwelling elderly individuals: a longitudinal study. J Frailty Aging. 2015;4(2):50-55. doi: 10.14283/jfa.2015.43. [DOI] [PubMed] [Google Scholar]
- 46. Gill TM, Gahbauer EA, Han L, Allore HG. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci. 2011;66(11):1238-1243. doi: 10.1093/gerona/glr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hall YN, Larive B, Painter P, et al. Effects of six versus three times per week hemodialysis on physical performance, health, and functioning: Frequent Hemodialysis Network (FHN) randomized trials. Clin J Am Soc Nephrol. 2012;7(5):782-794. doi: 10.2215/CJN.10601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaysen GA, Larive B, Painter P, et al. Baseline physical performance, health, and functioning of participants in the Frequent Hemodialysis Network (FHN) trial. Am J Kidney Dis. 2011;57(1):101-112. doi: 10.1053/j.ajkd.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reese PP, Cappola AR, Shults J, et al. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013;38(4):307-315. doi: 10.1159/000355568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hartmann EL, Kitzman D, Rocco M, et al. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4(3):588-594. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lorenz EC, Cheville AL, Amer H, et al. Relationship between pre-transplant physical function and outcomes after kidney transplant. Clin Transplant. 2017;31(5). doi: 10.1111/ctr.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nastasi AJ, McAdams-DeMarco MA, Schrack J, et al. Pre-kidney transplant lower extremity impairment and post-kidney transplant mortality. Am J Transplant. 2018;18(1):189-196. doi: 10.1111/ajt.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59(6):M627-M632. [DOI] [PubMed] [Google Scholar]
- 54. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738-743 [DOI] [PubMed] [Google Scholar]
- 55. Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127(5):494-496. doi: 10.1016/j.mad.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 57. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53-61. doi: 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Walker SR, Brar R, Eng F, et al. Frailty and physical function in chronic kidney disease: the CanFIT study. Can J Kidney Health Dis. 2015;2:32. doi: 10.1186/s40697-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10-12. doi: 10.1093/ageing/aft160. [DOI] [PubMed] [Google Scholar]
- 60. Shafi S, Zimmerman B, Kalil R. Temporary inactive status on renal transplant waiting list: causes, risk factors, and outcomes. Transplant Proc. 2012;44(5):1236-1240. doi: 10.1016/j.transproceed.2012.01.126. [DOI] [PubMed] [Google Scholar]
- 61. Li B, Cairns JA, Draper H, et al. Estimating health-state utility values in kidney transplant recipients and waiting-list patients using the EQ-5D-5L. Value Health. 2017;20(7):976-984. doi: 10.1016/j.jval.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19(5):841-843. doi: 10.1681/ASN.2007121354. [DOI] [PubMed] [Google Scholar]
- 63. Ambler G, Omar RZ, Royston P. A comparison of imputation techniques for handling missing predictor values in a risk model with a binary outcome. Stat Methods Med Res. 2007;16(3):277-298. doi: 10.1177/0962280206074466. [DOI] [PubMed] [Google Scholar]
- 64. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. [Google Scholar]
- 65. Li J, Scheike TH, Zhang MJ. Checking fine and gray subdistribution hazards model with cumulative sums of residuals. Lifetime Data Anal. 2015;21(2):197-217. doi: 10.1007/s10985-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Q, Tang G, Costantino JP, Chang CCH. Robust prediction of the cumulative incidence function under non-proportional subdistribution hazards. Canadian J Stat. 2016;44:127-141. [Google Scholar]
- 67. Zheng Y, Parast L, Cai T, Brown M. Evaluating incremental values from new predictors with net reclassification improvement in survival analysis. Lifetime Data Anal. 2013;19(3):350-370. doi: 10.1007/s10985-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Anderson PGR. Cox’s regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100-1120. [Google Scholar]
- 69. Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11(5):917-922. [DOI] [PubMed] [Google Scholar]
- 70. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, FrailtyProtocol2019Supplemental_Files for Frailty and the Kidney Transplant Wait List: Protocol for a Multicenter Prospective Study by Karthik K. Tennankore, Lakshman Gunaratnam, Rita S. Suri, Seychelle Yohanna, Michael Walsh, Navdeep Tangri, Bhanu Prasad, Nessa Gogan, Kenneth Rockwood, Steve Doucette, Laura Sills, Bryce Kiberd, Tammy Keough-Ryan, Kenneth West and Amanda Vinson in Canadian Journal of Kidney Health and Disease