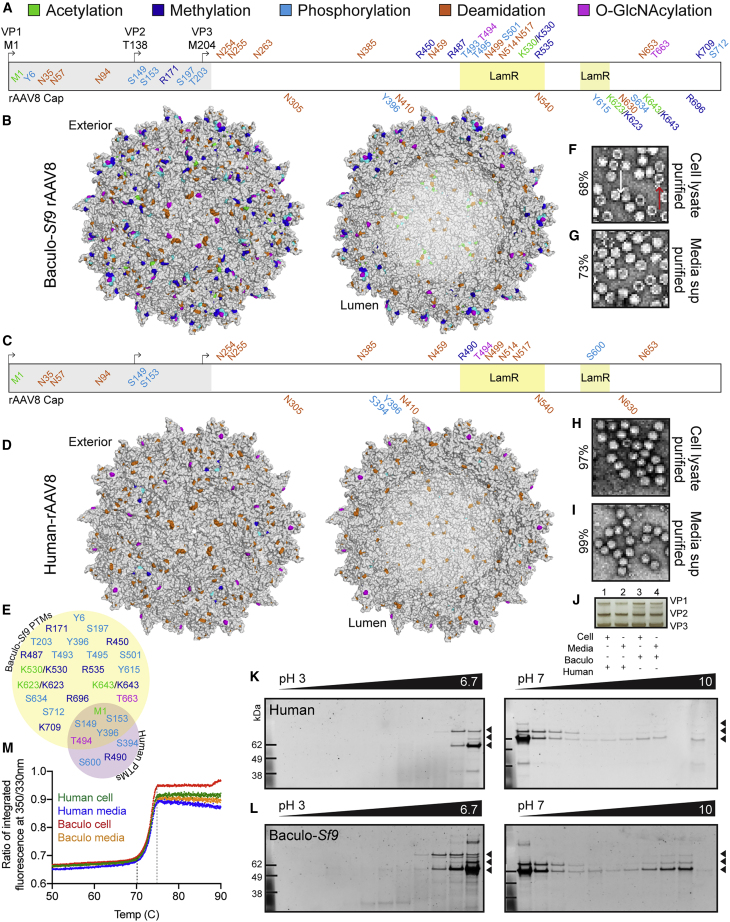
Figure 1.
rAAV Capsids Manufactured with the Human and Baculovirus-Sf9 Platforms are Post-Translationally Modified and Exhibit Differential PTM Profiles
(A) PTM identities and residue positions along the length of the rAAV8 polypeptide from the N to C terminus in the baculovirus (baculo-)Sf9 vector. PTMs are colored by type (acetylation, green; methylation, blue; phosphorylation, cyan; deamidation, orange; O-GlcNAcylation, magenta). Residues above the sequence are externally facing on the capsid. Residues below are lumenal or buried. Residues within the gray box from 1 to 220 represent the disordered region of AAV8 yet to be crystallized. The two regions for LamR binding (491–547 and 593–623) are highlighted in yellow boxes. (B) Cumulative capsid PTMs observed from all baculo-Sf9 rAAV8 lots, purified from both cell lysates and media. Same color code as in (A). (C) Same as (A) but with human-produced rAAV8. (D) Same as (B) but with human rAAV8. (E) Shared and unique capsid PTMs for rAAV8 produced in the baculo-Sf9 (yellow) and human (purple) platforms. Same color code as in (A). Excluded are deamidation degradation marks which are universal. (F) Negative staining and TEM imaging of baculo-Sf9 rAAV8 cell-purified vector. White arrow indicates full capsid; red arrow indicates empty capsid; for reference for (F)–(I) (percent full capsids noted on left). Original magnification, ×20,000. (G) Same as (F) but media-purified vector. (H) Same as (F) but with human rAAV8 cell-purified vector. (I) Same as (H) but with media-purified vector. (J) Silver stain of capsid VP species present in vector lots from (F)–(I). (K) 2D gel images from human-produced rAAV8 from pH 3 to pH 10. VP1 (87 kDa), VP2 (72 kDa), and VP3 (62 kDa) bands are indicated with black arrowheads. (L) 2D gel images from baculo-Sf9-produced rAAV8. (M) Thermal capsid melt curves for rAAV8 vectors shown from 50°C to 90°C; full melt curves from 30°C to 95°C are in Figure S7A. Tm initiation, dashed black line; final Tm, dashed gray line.