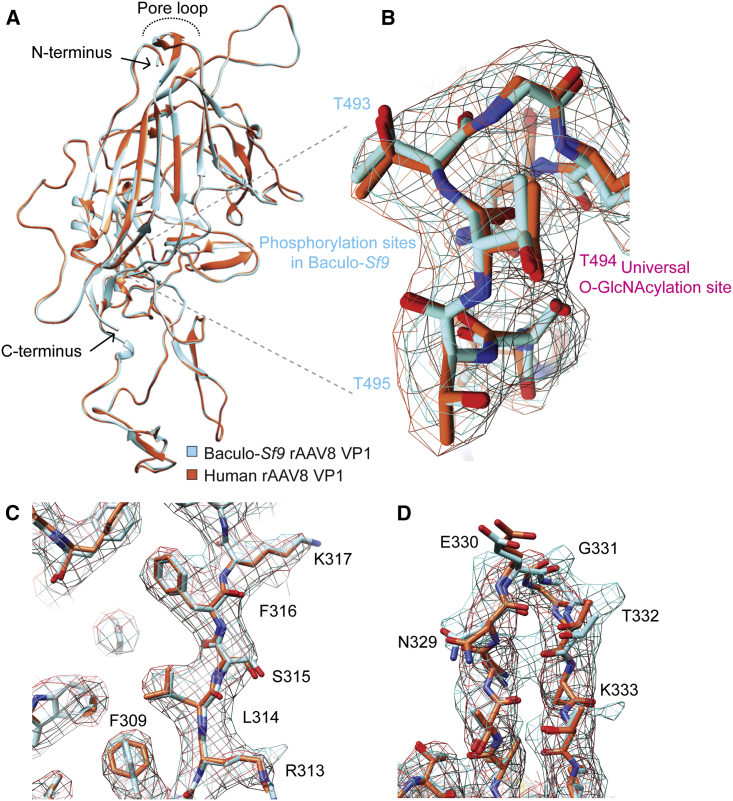
Figure 2.
Human and Baculovirus-Sf9 Production Platforms Produce rAAV Capsids with Similar Structures by Cryo-EM
(A) Overlay of an individual VP1 chain from human (red-orange) and baculovirus (baculo-)Sf9 (cyan) full rAAV8 capsids highlighting an absence of macro-level differences. Key structural landmarks are shown. (B) Magnified overlay of the LamR binding region from a human (red-orange electron density caging) and baculo-Sf9 (cyan) full rAAV8 capsid highlighting residues capable of post-translational modification as determined by LC-MS/MS. (C) Magnified overlay depicting potential side-chain level structural differences in phenyl ring orientations between human and baculo-Sf9 rAAV8. (D) Magnified overlay of a single human and baculo-Sf9 rAAV8 capsid cylinder loop depicting minor potential side-chain level differences.