Abstract
Objective
Loss of smell and taste are considered potential discriminatory symptoms indicating triaging for coronavirus disease 2019 (COVID-19) and early case identification. However, the estimated prevalence essential to guide public health policy varies in published literature. This meta-analysis aimed to estimate prevalence of smell and taste loss among COVID-19 patients.
Data Sources
We conducted systematic searches of PubMed, Embase, Web of Science, and Google Scholar databases for studies published on the prevalence of smell and taste loss in COVID-19 patients.
Review Methods
Two authors extracted data on study characteristics and the prevalence of smell and taste loss. Random-effects modeling was used to estimate pooled prevalence. Subgroup analysis and meta-regression were conducted to explore potential heterogeneity sources. This study used PRISMA and MOOSE guidelines.
Results
Twenty-seven of 32 studies reported a prevalence of loss of smell, taste, or both from a combined sample of 20,451 COVID-19 patients. The estimated global pooled prevalence of loss of smell among 19,424 COVID-19 patients from 27 studies was 48.47% (95% CI, 33.78%-63.29%). Loss of taste was reported in 20 studies and 8001 patients with an estimated pooled prevalence of 41.47% (95% CI, 3.13%-31.03%), while 13 studies that reported combined loss of smell and taste in 5977 COVID-19 patients indicated a pooled prevalence of 35.04% (95% CI, 22.03%-49.26%).
Conclusions
The prevalence of smell and taste loss among COVID-19 patients was high globally, and regional differences supported the relevance of these symptoms as important markers. Health workers must consider them as suspicion indices for empirical diagnosis of severe acute respiratory syndrome coronavirus 2 infection.
Keywords: COVID-19, coronavirus infection, severe acute respiratory syndrome, SARS-CoV-2, anosmia, gustatory loss, olfactory loss
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2, also referred to as nCov-2) was first reported in Wuhan, China, in December 2019; was declared a pandemic by the World Health Organization (WHO) 3 months later; and currently is spreading exponentially across over 200 countries of the world.1 It is a respiratory syncytial disease that is like SARS and Middle East respiratory syndrome (MERS-CoV). The natural history of the causative agent SARS-COv-2 is still evolving, including the mode of presentations. Major symptoms such as fever, rhinorrhea, sore throat, weakness/fatigue, abdominal pain, diarrhea, loss of appetite, cough, headache, and worsening difficulty with breathing have been documented.2 The nasal cavities and oropharynx are a common portal of entry, and it is known that the viral organism gets adapted and multiplies therein before being propagated to the lower respiratory tract, where the pathological damages and sequalae occur. Respiratory complications such as pneumonia, lung fibrosis, and death have also been reported in severe cases.2
Coronavirus is a contagious, virulent organism with an incubation period ranging from 2 to 14 days within which asymptomatic patients can transmit infection. It is difficult to identify carriers and asymptomatic patients during the incubation period. Therefore, stringent measures are currently being applied, including social distancing, handwashing, wearing of face masks, total lockdown of cities and countries, aggressive contact tracing, and community testing all in the bid to prevent, control, and contain the spread of the disease.1,2 Identification of early symptoms and signs that could indicate COVID-19 infections is a veritable tool for triaging and early identification of patients.3
Several studies from the United States, China, South Korea, Iran, Australia, France, Spain, Italy, United Kingdom, and Germany strongly confirmed different degrees of loss of smell (anosmia/hyposmia/dysosmia) and loss of taste (ageusia/hypogeusia/dysgeusia) as early symptoms of COVID-19.4-32 It was observed that sudden loss in smell and taste could be the only features in asymptomatic newly infected individuals and could also serve as early symptoms of the disease. In fact, the American Academy of Otolaryngology–Head and Neck Surgery Foundation and Ear, Nose, and Throat Society of the United Kingdom (ENTUK) recommended that patients presenting with these clinical features should commence self-isolation before alerting the health authorities.33,34 Despite the many opinions of ear, nose, and throat (ENT) and public health experts on the importance of loss of smell and taste, it was only sometime in May 2020 that the WHO began to recognize them as key symptoms of COVID-19.35
Information from individual published reports from many countries on the association of loss of smell and taste with COVID-19 has accumulated over a short period of time, but evidence remains controversial as emerging estimates of pooled prevalence come from relatively small studies.28,36,37 The few accessible literature reviews on loss of smell and taste in COVID-19 patients do not include a meta-analysis that should provide global or regional pooled estimates.36,37 There has also been an increase in published studies since these reviews appeared in the literature. To this end, we conducted a systematic review and meta-analysis of data on loss of smell and/or taste loss in COVID-19 patients to determine global and regional prevalence.
Methods
Data Sources and Search Strategy
We searched for published studies that reported findings on abnormalities of smell and taste in patients with “acute respiratory coronavirus 2 (SARS-CoV-2)” infection or COVID-19 using PubMed, Embase, Web of Science, and Google scholar (https://scholar.google.com). These databases were searched for studies with data on the incidence or prevalence of loss of smell and/or taste between December 2019 and May 2020. The studies were restricted to only those studies involving human subjects and written in English. The search strategy used the exploded Medical Subject Headings terms and text words: ((((((“COVID-19”)) OR (“COV-2”)) OR (“Corona virus 2”)) OR (coronavirus)) AND (((((“loss of smell”) OR (Anosmia)) OR (Hyposmia)) OR (olfaction)) OR (“olfactory loss”))) AND ((((((ageusia) OR (Hypogeusia)) OR (dysgeusia)) OR (“gustatory loss”)) OR (gustation)) OR (“loss of taste”)). In addition, we searched some reference lists of relevant articles by hands to identify further relevant literatures, but no additional articles were found. The details of terms used and the search steps are presented in Supplemental Tables S1 and S2 (in the online version of the article). We also imported the relevant articles to EndNote X8 and deleted duplicate publications from the databases.
Study Selection and Eligibility Criteria
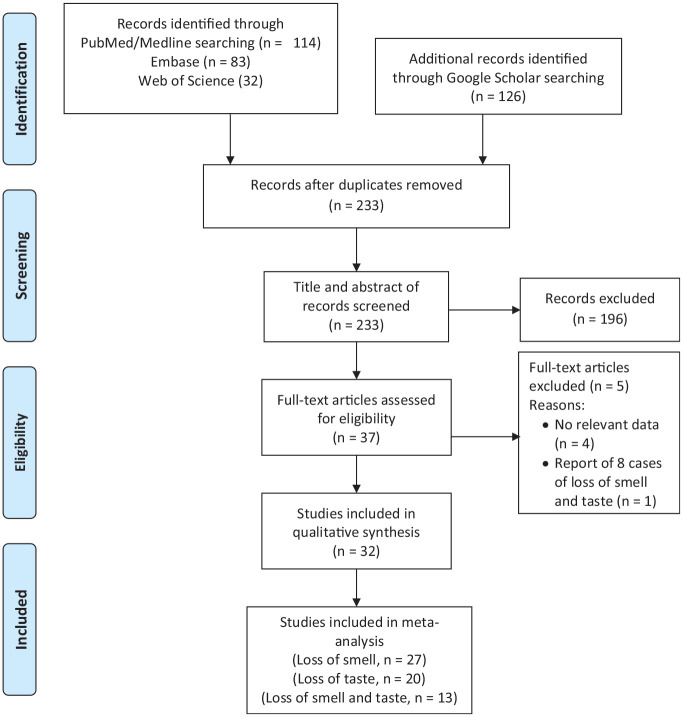
We included published journal articles that reported data on any incidence or prevalence of loss of smell and/or taste in COVID-19 patients. The titles and abstracts of the articles selected for the studies with objectives/focus on the desired results were further reviewed in details. The steps followed in the selection process were as shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram ( Figure 1 ). The decision to include a study was based on the presence of data on loss of smell and taste in COVID-19 patients in the abstract or in the body of the article. Subsequently, each eligible article was read to fully identify the relevant data. Only studies that met the inclusion criteria were reviewed and analyzed. We recognized that different researchers used different case definitions for smell and taste loss. We have therefore defined our outcome of interest as a partial or complete loss of smell, taste, or both. Thus, the 3 results examined in this systematic review were “partial or complete loss of smell,”“partial or complete loss of taste,” and “concurrent partial or complete loss of smell and taste.”
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for study selection and exclusion.
Inclusion and exclusion criteria
We have included studies that investigated or described the mechanism of loss of smell and taste, the prevalence of loss of smell and taste, and the stage of occurrence of loss of smell and taste in patients with diagnosis of COID-19. Conversely, studies that were published as letters to the editor, conference proceedings, and editorials as well as animal studies were excluded from review and meta-analysis.
Assessment of Quality of Studies
Three reviewers separately (T.S.I., A.J.F., and A.E.O.) reviewed the titles and abstracts to provide full-text reviews of studies. The quality of the studies was evaluated using the standard assessment criteria of the Joanna Briggs Institute (JBI)38 in cohort and cross-sectional studies and case series. The following elements were used: (1) appropriateness of inclusion criteria; (2) description of study subject and setting; (3) valid and reliable measurement of exposure; (4) objective, standard criteria; (5) identification of confounders; (6) strategies for handling confounders; (7) outcome measurement; and (8) appropriateness of statistical analysis. Studies of the quality scale that exceeded 50% and higher were considered as low risk of bias. Any disparities were resolved by consensus.
Data Extraction
The review was carried out following the MOOSE (Meta-analyses Of Observational Studies in Epidemiology) checklist. The database abstraction proforma was developed after iterative database searches and screenings for all titles and abstracts to find full-text studies for a thorough analysis. Data were extracted, and all data collected using Microsoft Excel 2016 were reconciled by 2 authors (TSI and AJF). The third author (A.E.O.) compared the extracted information to the original data reported in the selected full text (or in the documentation provided by the authors) to ensure the accuracy of the extracted data. Where necessary, any identified errors were discussed and corrected.
Data analysis
The extracted data were entered into Microsoft Excel and analyzed using Stata/SE 12.1 for Windows (StataCorp LP). After quality assessment, the included studies were subjected to meta-analysis using “metaprop,”“metafunnel,” and “metabias.” We used the random-meta-analysis model of weighted inverse variances to obtain an overall summary assessment of the prevalence across studies. A sensitivity analysis for the consistency of the summary estimate was conducted. The bias in publication was assessed using funnel plots and Egger’s linear regression test. The I2 statistics also measured the heterogeneity of the studies. In addition, publication bias was investigated using the trim-and-fill analysis (funnel plots). We presented the results of subgroup analysis by study designs and continents where studies were carried out.
Results
Characteristics of the Studies Included in the Review
A total of 240 articles were identified through literature searches. Figure 1 shows the study selection and exclusion flowchart. After removal of duplicates, 233 articles were screened by title and abstract, and 37 were found to be eligible for full-text assessment. Of these full-text articles, 32 studies with a total of 20,451 COVID-19 patients were suitable for meta-analysis. The key features and results of the evaluation of risk of bias for the included studies are summarized in Table 1 . The included studies were conducted in 13 countries. Although 7 (21.9%) and 6 (18.8%) of the studies included in this review were published in Italy and France, contributing 1609 and 2793 to the study population, respectively, the United Kingdom, with 3 studies, contributed the largest number (9988) to the sample size. Of the 32 studies included in the meta-analysis, 27 reported loss of smell, 20 reported loss of taste, and 13 reported both loss of smell and taste as a single entity. Regarding the study designs, there were 20 cross-sectional studies, 6 prospective cohorts, 3 case-control studies, and 3 retrospective analyses of hospital records. The JBI quality appraisal checklists indicated that 29 were rated good while 3 were of fair quality. The study sample size and outcome reported in the studies are also presented in Table 1 .
Table 1.
Characteristics and Outcomes of Assessment of Risk of Bias for the Included Studies.
| First author | Study design | Country | Qualitya | Sample size | Instrument used |
|---|---|---|---|---|---|
| Abalo-Lojo5 | Cross-sectional | Spain | Good | 131 | Self-reporting questionnaire |
| Aggarwal6 | Retrospective | United States | Good | 16 | Questionnaire |
| Beltrán-Corbellini7 | Case-control | Spain | Good | 79 | Questionnaire |
| Bénézit39 | Cross-sectional | France | Good | 68 | Web-based questionnaire |
| Carignan8 | Case-control | Canada | Good | 134 | Telephone interview questionnaire |
| Coelho9 | Cross-sectional | United States | Good | 93 | Web-based survey |
| Giacomelli48 | Cross-sectional | Italy | Good | 59 | Questionnaire |
| Gudbjartsson40 | Cross-sectional | Iceland | Good | 1221 | Questionnaire |
| Hopkins12 | Cross-sectional | United Kingdom | Good | 382 | Online survey |
| Hopkins41 | Cross-sectional | United Kingdom | Good | 2428 | Survey |
| Kim13 | Cross-sectional | South Korea | Good | 213 | Questionnaire based survey |
| Klopfenstein14 | Retrospective | France | Fair | 114 | Medical files |
| Lechien42 | Cross-sectional | France | Good | 86 | Nutritional health and nutritional examination survey |
| Lechien43 | Cohort | France | Good | 417 | Questionnaire |
| Lechien44 | Cohort | France | Good | 2013 | Sniffin’ sticks and questionnaire |
| Lee17 | Cross-sectional | South Korea | Good | 3191 | Telephone interview |
| Levinson45 | Cross-sectional | Israel | Good | 42 | Questionnaire and phone interview |
| Liguori19 | Cross-sectional | Italy | Good | 103 | Anamnestic interview |
| Liu20 | Cohort | Taiwan | Good | 321 | Open access data |
| Mao21 | Cross-sectional | China | Good | 214 | Electronic medical records |
| Menni22 | Cross-sectional | United Kingdom | Good | 7178 | Smartphone based app |
| Merza23 | Cross-sectional | Iraq | Fair | 15 | Questionnaire |
| Moein24 | Cohort | Iraq | Good | 60 | Pennsylvania Smell Identification Test |
| Paderno46 | Cross-sectional | Italy | Good | 508 | Survey-based questionnaire |
| Speth26 | Cross-sectional | Switzerland | Good | 103 | Telephone questionnaire |
| Spinato27 | Cohort | Italy | Good | 202 | Questionnaire |
| Vaira47 | Cohort | Italy | Good | 345 | Connecticut Chemosensory Clinical Research Center Orthonasal Olfaction Test |
| Vaira49 | Cross-sectional | Italy | Good | 72 | Connecticut Chemosensory Clinical Research Center Orthonasal Olfaction Test |
| Vaira29 | Cross-sectional | Italy | Good | 320 | Medical records |
| Yan50 | Case-control | United States | Good | 59 | Internet-based platform |
| Yan31 | Cross-sectional | United States | Good | 169 | Self-reported questionnaire |
| Zayet32 | Retrospective | France | Fair | 95 | Questionnaire |
Quality rated as poor, fair, or good.
Prevalence of Loss of Smell and/or Taste in COVID-19 Patients
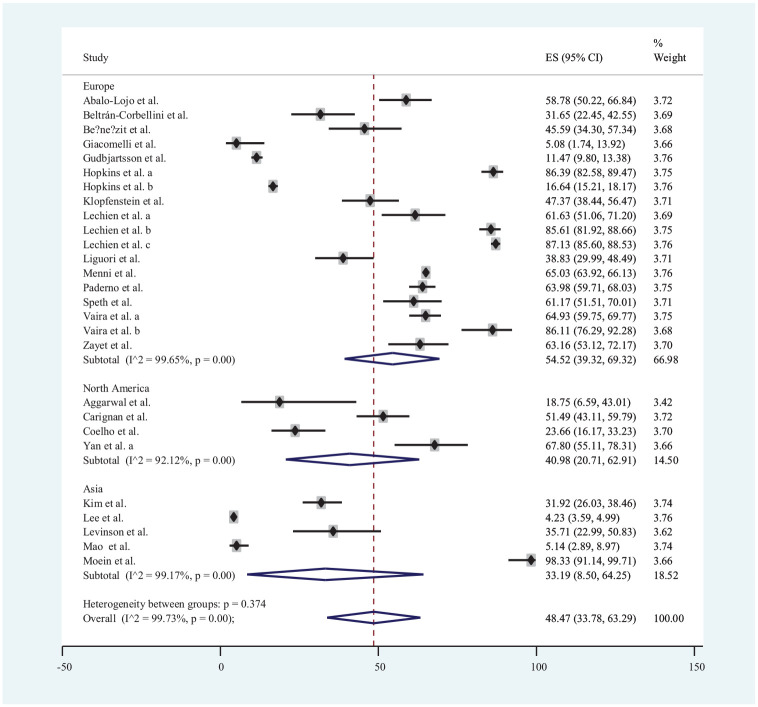
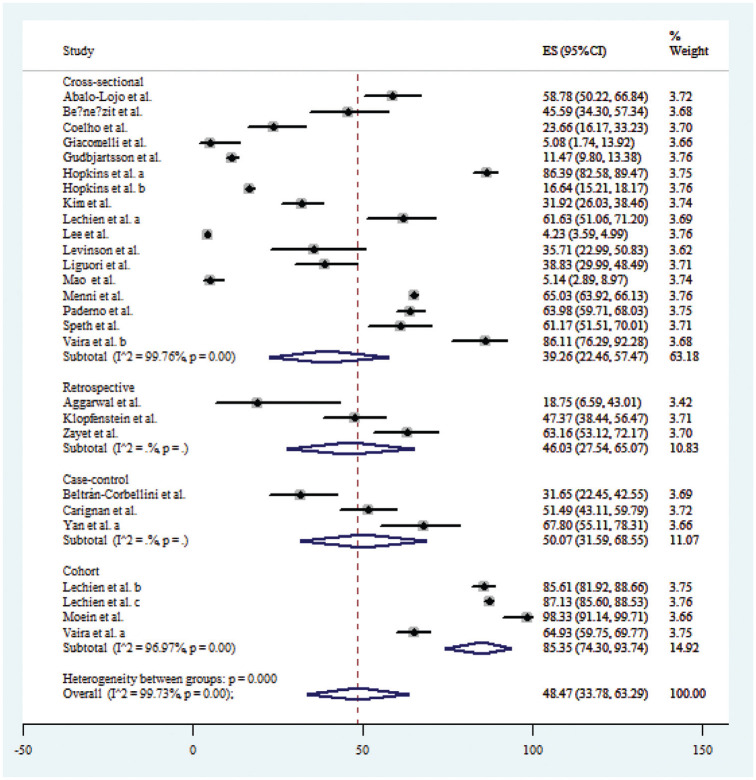
Twenty-seven publications* that reported data on loss of smell were used for estimation of its prevalence with a total of 19,424 COVID-19 patients. Accordingly, the prevalence of loss of smell among COVID-19 patients ranged from 4.23% in Lee et al17 to 98.33% in Moein et al.24 The estimated global pooled prevalence of loss of smell among COVID-19 patients was 48.47% (95% CI, 33.78%-63.29%). There were 6 publications from Asia,7,13,17,21,24,45 17 from Europe,† and 4 from North America6,8,9,50 that reported data on loss of smell. The estimated pooled prevalence of loss of smell in the populations of Asia, Europe, and North America regions was 54.52% (95% CI, 39.32%-69.32%), 40.98% (95% CI, 20.71%-62.91%), and 33.19% (95% CI, 8.50%-64.25%), respectively ( Figure 2 ).
Figure 2.
Forest plot for loss of smell in COVID-19 patients.
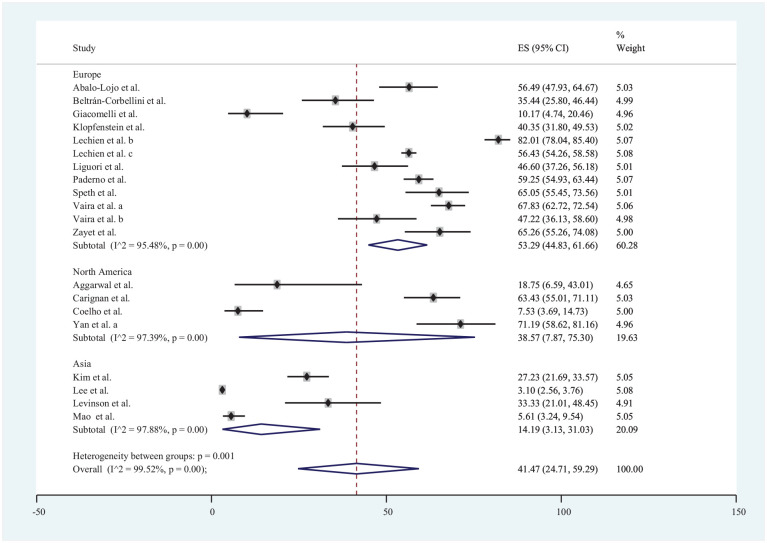
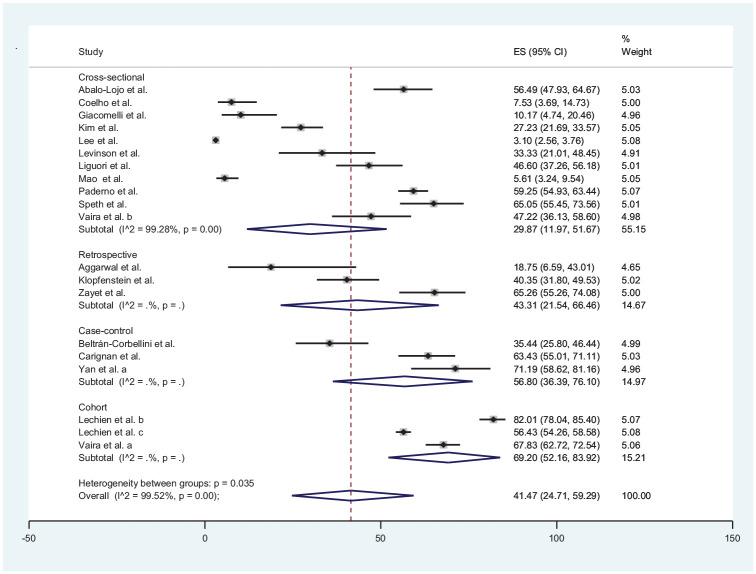
Loss of taste was reported in 20 studies‡ included in the review with data from a total of 8001 patients. The estimated prevalence of COVID-19 ranged from 3.10% in Lee et al17 to 82.01% in Lechien et al.43 The estimated pooled prevalence of loss of taste in COVID-19 patients was 41.47% (95% CI, 3.13%-31.03%). Loss of taste was reported in 5 publications from Asia,7,13,17,21,45 11 from Europe,§ and 4 from North America.6,8,9,52 The estimated pooled prevalence of loss of taste in the populations of Europe, North America, and Asia regions was 53.29% (95% CI, 44.83%-61.66%), 38.57% (95% CI, 7.87%-75.30%), and 14.19% (95% CI, 3.13%-31.03%), respectively ( Figure 3 ).
Figure 3.
Forest plot for loss of taste in COVID-19 patients.
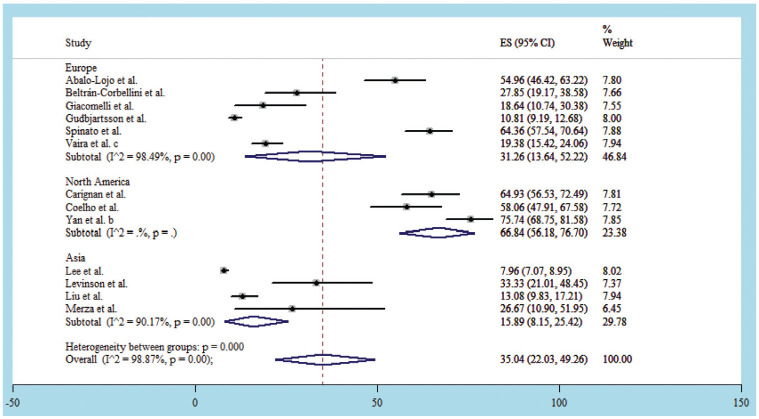
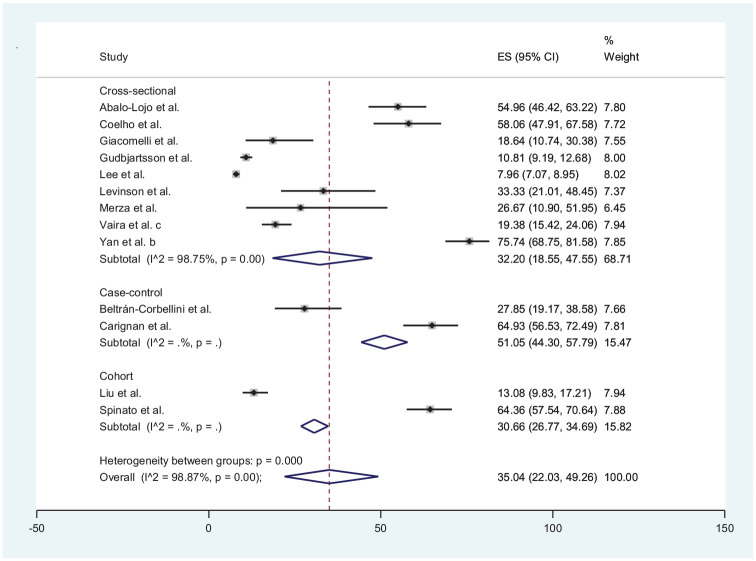
Thirteen studies║ reported combined loss of smell and taste with data from a total of 5977 COVID-19 patients. The estimated prevalence of COVID-19 in the 13 studies ranged from 7.96% by Lee et al17 to 75.74% Yan et al.31 The estimated pooled prevalence of combined loss of smell and taste in COVID-19 patients was 35.04% (95% CI, 22.03%-49.26%). Both loss of smell and taste were reported in 5 publications each from Asia7,17,20,23,45 and Europe5,27,40,48,49 and 3 from North America.8,9,31 The estimated pooled prevalence of loss of smell and taste in the populations of Europe, North America, and Asia regions was 31.26% (95% CI, 13.64%-52.22%), 66.84% (95% CI, 56.18%-76.70%), and 15.89% (95% CI, 8.15%-25.42%), respectively ( Figure 4 ).
Figure 4.
Forest plot for loss of smell and taste in COVID-19 patients.
Homogeneity and sensitivity analysis
The heterogeneity assessment of the 27 studies that reported data on loss of smell is shown in Figure 2 . The I2 statistics showed that the studies were significantly heterogenous within the groups with variation above 99%, but there was no significant heterogeneity between groups (P = .374). However, the sensitivity analysis of the data showed, however, that the sizes of effects in the studies are similar despite the successive omission and that the 95% CIs significantly overlap (see Suppl. Table 3 in the online version of the article).
Similarly, the forest plot from meta-analysis of data from 20 studies on loss of taste shown in Figure 3 indicates that the studies were substantially heterogeneous, and the I2 statistics indicate that the estimated variations ranged from 95.48% among studies conducted in Europe to 97.88% in those published from Asia. Overall, the pooled estimated heterogeneity was 99.52% (P < .001). Nevertheless, the sensitivity analysis of the data on loss of smell showed that effect study sizes across the studies were comparable with consecutive omission of study and that 95% CIs significantly overlapped (see Suppl. Table S4 in the online version of the article).
For studies that reported both loss of smell and taste, the analysis of 13 studies significantly showed heterogeneity, and the overall variation was estimated to be about 98.87% (I2); P < .001. However, the sensitivity analysis of the data revealed that the effect sizes were similar across the studies with consecutive omission, and the 95% CIs overlapped considerably (see Suppl. Table S5 in the online version of the article).
Further subanalysis by study designs revealed that cross-sectional (I2 = 99.76%; P < .001), retrospective (I2 = 82.5; P = .003), case-control (I2 = 88.5.4; P < .001), and cohort studies (I2 = 96.97; P < .001) for loss of smell were significantly heterogeneous ( Figure 5 ). Similar results were also obtained for the subanalyses by study designs for loss of taste ( Figure 6 ) and a combination of loss of smell and taste ( Figure 7 ). The extent of the heterogeneity varied significantly across the 4 types of study designs.
Figure 5.
Forest plot of loss of smell by types of study design.
Figure 6.
Forest plot of loss of taste by types of study design.
Figure 7.
Forest plot of loss of smell and taste by types of study design.
Assessment of publication bias
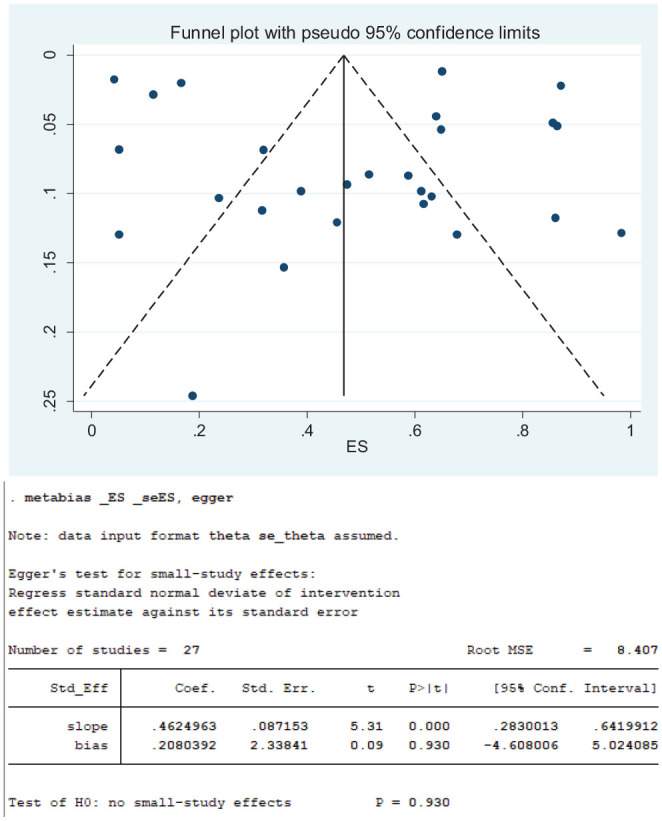
As displayed on the funnel plot in Figure 8 , the distribution of the 27 studies that reported loss of smell in COVID-19 patients appears symmetrical, indicating absence of bias. This observation was also corroborated by Egger’s and Begg’s tests, which showed that there was no statistically significant publication bias (P = .930). Although the distribution of the 20 studies that reported loss of taste appears asymmetrical, there were more studies on the right side of the vertical middle line ( Figure 9 ), and Egger’s and Begg’s tests suggest that there was no statistically significant publication bias (P = .167). Contrary to observations of an absence of publication biases among studies that reported loss smell and loss of taste, the distribution of the 13 studies that reported concurrent loss of smell and taste indicates remarkable publication bias ( Figure 10 ). Notably, there were more studies on the right side of the vertical middle line than on the left, and a considerably small-size study is extremely close to the effect size (ES) axis. Egger’s and Begg’s tests also indicated significant bias (P = .006).
Figure 8.
Funnel plots for loss of smell.
Figure 9.
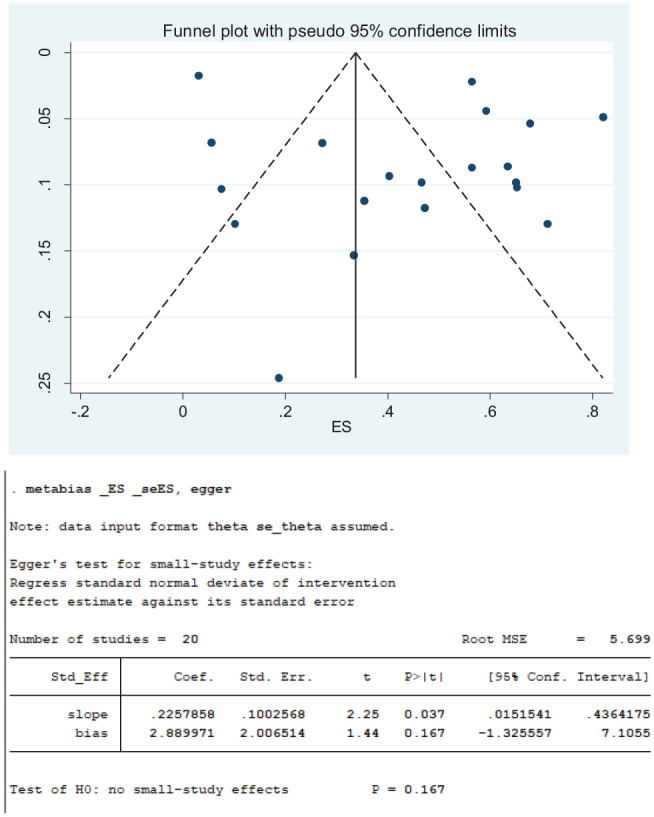
Funnel plots for loss of taste.
Figure 10.
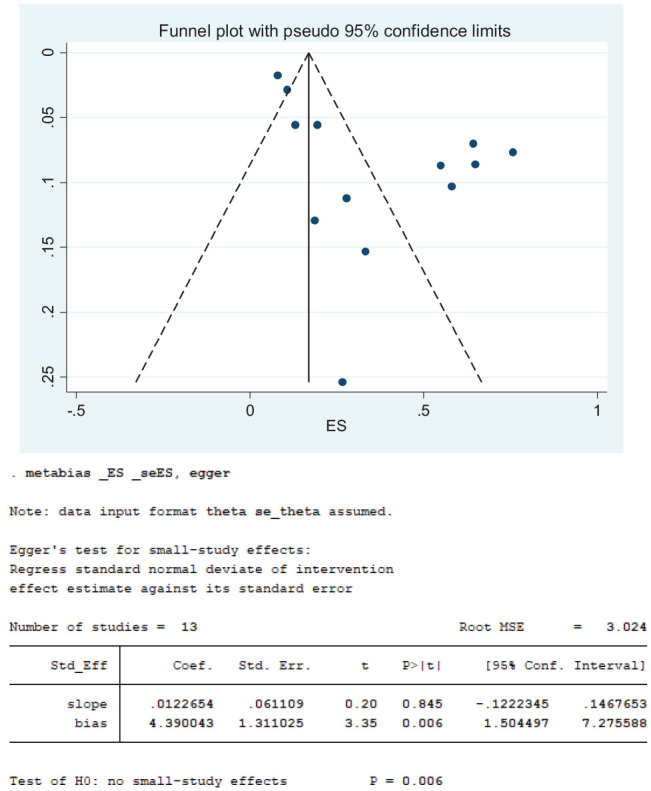
Funnel plots for loss of smell and taste.
Discussion
This systematic review revealed that a remarkable number of studies have investigated loss of smell and taste across 3 continents over a period of 5 months in 2020 with obvious lack of quantitative data from Australia, Africa, and South America ( Table 1 ). There were more studies from Europe than from the remaining 2 continents with data on loss of smell and taste in COVID-19. Our search, limited to only studies published in English, might have restricted inclusion of studies published in other languages. However, there appeared to be no study from the African continent, and this might be a reflection of poor responses of scientists to the pandemic in the region.
Since inception of the COVID-19 pandemic, reports on the burden of olfactory and gustatory dysfunctions in COVID-19 patients are still evolving. To our knowledge, up to the time of this writeup, there were only 3 published systematic reviews with meta-analyses of individual data on the subject. The estimated global pooled prevalence of loss of smell among 19,424 COVID-19 patients from 27 published articles included in this present study was 48.47% (95% CI, 33.78%-63.29%). This estimate is slightly lower than the prevalence reported by Tong et al28 (52.73%) and Costa et al36 (60.7%), which included only 10 and 6 publications, respectively, with relatively smaller sample sizes. Conversely, Agyeman et al53 obtained a global estimate of 41.0% from 24 studies involving a study population of 8438, which is comparatively lower than the values obtained in the present study.
Our review revealed that loss of taste was a less frequently reported symptom than loss of smell in COVID-19 patients with an estimated pooled prevalence of 41.47% (95% CI, 3.13%-31.03%). This estimate is comparable with 38.2% by Agyeman et al53 but lower than 43.93% and 56.4% by Tong et al28 and Costa et al,36 respectively. It is worth noting that the number of articles and study population involved in the present review were remarkably larger than all the 3 earlier published systematic reviews with meta-analyses on loss of smell and taste in COVID-19 patients.28,36,53
One major difference between the present study and other available and accessible similar systematic reviews28,36,53 is that we included studies that investigated prevalence of combined loss of smell and taste. To this end, our study showed that the estimated pooled prevalence of combined loss of smell and taste in COVID-19 patients was 35.04% (95% CI, 22.03%-49.26%). This value was obtained from 13 studies that involved 5977 COVID-19 patients. These 13 studies included 5 each from Asia and Europe and 3 from North America. The combination of loss of smell and taste was less reported in the literature, as there were no data for the remaining 19 of 32 studies included in this review.
Overall, this review has shown that loss of smell and taste, either singly or combined, are important clinical features of COVID-19 as at least 1 of every 3 affected individuals is likely to exhibit any of these symptoms. Our data clearly corroborate the earlier position of various researchers4-32 and the later adoption by the WHO as important discriminatory symptoms of COVID-19.35 In addition to the 3 most common symptoms (fever, dry cough, and tiredness) listed by the WHO, clinicians or health care providers in countries where COVID-19 spread persists should constantly pay attention to these symptoms.35 The clinical implication of this is that the chance of detection and suspicion index of health workers in resource-limited countries where access to the COVID-19 test is limited will increase by paying attention to sudden loss of smell and/or taste. The ENT Society of United Kingdom hypothesized that patients with only anosmia, who do not meet the criteria for self-isolation, might be hidden carriers or “vectors” that have potential to facilitate rapid spread of COVID-19 in the community.33 The American Academy of Otolaryngology–Head and Neck Surgery has suggested the possibility of COVID-19 infection in individuals with sudden anosmia/hyposmia and ageusia/dysgeusia in the absence of other respiratory symptoms such as nasal obstruction and rhinorrhea.34 It was suggested that such individual(s) should self-isolate for 14 days to reduce the number of hidden carriers capable of spreading the infection.
The exact pathophysiology of the loss of smell and taste in coronavirus infection is yet to be fully understood. Some authors have postulated that the SARS-CoV-2 virus infects cells through interactions between its spike (S) protein and the angiotensin-converting enzyme 2 (ACE2) protein receptor on the target cells likely by the cell surface protease transaminase protease serine 2 (TMPRSS2).54,55 The respiratory epithelium and its supporting olfactory cells harbor the highest concentration of ACE2 and TMPRSS2 receptors and therefore appear to serve as a reservoir for replication of the SARS-CoV-2 virus.
The proximity of the respiratory epithelial cells (chief reservoir site), the supporting olfactory cells (second most susceptible site), and the olfactory bulb highly suggests brevity in the period of exposure to infection and involvement of the olfactory bulb, leading to paresis or paralysis in sense of smell. This may support the hypothesis of early dysfunctional sense of smell and taste in COVID-19. Patients with severe hyposmia or anosmia usually complain of loss of taste, as reported by most articles included in this systematic review and meta-analysis. This is due to loss in the contribution of smell to their perception of flavor. One could argue that hypogeusia or ageusia in COVID-19 is due to hyposmia or anosmia rather than true pathologic hypogeusia/ageusia. This evidence could also explain the underlying pathogenic mechanism of smell and taste loss in COVID-19.
There are 3 major postulated mechanisms by which viruses affect smell. First, viral infection of the nasal mucosa could trigger inflammation of both respiratory and olfactory mucosa to create a barrier to odor chemicals (inhaled aromatic particles in the air) between the dendritic cells of the olfactory sensory neurons and olfactory mucosa, thereby leading to disruption in odor detection.56 The second mechanism is by direct virus attack on the olfactory receptors, causing their damage with resultant inhibition of transmission of odor signals. This leads to either temporal or permanent loss of smell.57,58 The third mechanism is that the virus, being neurotropic, could penetrate through the cribriform plate to infect the olfactory bulb and follow the olfactory pathway to attack the olfactory cortex of the temporal lobe of the brain responsible for smell. The coronavirus footprint has been found in the cerebrospinal fluid and brain of COVID-19 patients, thereby clinically verifying viral encephalitis.59 Any or all these 3 may be possible mechanism(s) for loss of smell and, by extension, taste in COVID-19, although this is still subject to further investigation.
The strength of this systematic review lies in the large number of included studies and size of the population as well as the geographical spread ( Figure 1 ). In addition, we have used both quantitative and qualitative methods to establish the validity and inclusiveness of the review. However, no attempt was made at exploring data that may explain the mechanisms or causality of loss of smell and taste in COVID-19 patients. We used a meta-analysis approach to estimate global and regional prevalence by pooling individual data from published studies ( Figures 2 - 4 ). The study validity was further strengthened by the subanalyses of the various studies according to the pooled study designs ( Figures 5 - 7 ) across the various geographical locations. We adopted the use of random-effect models because of the level of heterogeneity envisaged a priori, coupled with the fact that the included studies were adjudged to have fair to good quality in the assessment of bias ( Table 1 ). Table 1 also showed that only 3 of the 32 articles included in the meta-analysis used a quantitative validated test instrument to determine loss of smell and taste. This is understandable because the highly contagious and emerging nature of COVID-19 does not allow close contact testing for research at its early phase.
One issue that may limit generalization of our meta-analysis results is the statistical heterogeneity of the included studies, as demonstrated by the high variability values of greater than 80% in all the forest plots ( Figures 2 - 4 ). On visual inspection of the funnel plots ( Figures 8 - 10 ), the individual study effects vary remarkably, suggesting publication bias as a possible source of the observed heterogeneity, but the sensitivity analysis showed that the confidence intervals of all the studies consistently overlapped, and the effect sizes did not vary significantly with successive exclusion of studies (see Suppl. Tables S1-S5 in the online version of the article). The statistical heterogeneity in the results can be attributed to either clinical or methodological diversity or both. The heterogeneity could have been caused by variations in the studies in the regions. We mitigated the likelihood of data extraction errors as a potential cause of significant heterogeneity by involving 2 independent investigators in the identification and extraction of individual study data. Nevertheless, clinical variation and varying methods of smell and taste loss assessment may most likely have resulted in heterogeneity, but the patient characteristics are unlikely to have fully accounted for heterogeneity in the design of the study. Considerable statistical variation resulting from methodological variability or outcome estimation variations indicates that not all studies included estimate the same magnitude loss of smell or taste but do not inherently imply a significant variation in the prevalence. Another issue that might limit the external validity and precision of our estimated prevalence is the exclusion of articles not written in English. Given the fact that our review was done during the pandemic, it was impracticable to get quick and accurate translation and interpretation of articles written in languages other than English.
Although we conducted a comprehensive literature search using bibliographic databases and gray literature sources via Google Scholar and Africa Journal Online (AJOL), some unpublished articles and those not indexed in an electronic database linked to our intended sources of gray literature may have been inadvertently excluded. In the future, systematic reviews of smell and taste loss will need to consider the inclusion of publications written in languages other than English. It is conceivable that certain demographic and environmental characteristics as well as preexisting diseases such as hypertension and diabetes may influence the prevalence of loss of smell and/or taste, which were not captured in our review. Future study will need to consider investigation of the possibility of these associations of different factors related to prevalence of loss of smell and/or taste.
Conclusion
The prevalence of loss of smell and taste among COVID-19 patients was high globally and regionally. The observed magnitude of the reported loss of smell and taste among patients suggests that these symptoms are important markers of the disease. Therefore, health workers need to consider loss of smell and taste as indices of suspicion for empirical diagnosis of coronavirus infection.
Author Contributions
Titus Sunday Ibekwe, design, data collection, draft, revision, and accountability for work; Ayotunde James Fasunla, data collection, drafting, writing, revision, and final approval; Adebola Emmanuel Orimadegun, data analysis, design, writing, revision, and final approval.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Supplemental Material
Supplemental material, Supplemental_Tables_1_to_5 for Systematic Review and Meta-analysis of Smell and Taste Disorders in COVID-19 by Titus Sunday Ibekwe, Ayotunde James Fasunla and Adebola Emmanuel Orimadegun in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation
References 5-9, 12-14, 17, 19, 21, 22, 24, 26, 29, 31, 32, 39-48.
References 5, 12, 14, 19, 22, 26, 29, 32, 39-44, 46, 48, 49.
References 5-9, 13, 14, 17, 19, 21, 26, 29, 32, 43-45, 47, 48, 51, 52.
References 5, 14, 19, 26, 29, 32, 43, 44, 47, 48, 51.
References 5, 7-9, 17, 20, 23, 27, 31, 40, 45, 48, 49.
Supplemental Material: Additional supporting information is available at http://journals.sagepub.com/doi/suppl/10.1177/2473974X20957975
References
- 1. European Centre for Disease Prevention and Control (ECDC). COVID-19 situation update worldwide, as of 27 May 2020. Accessed May 28, 2020 https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
- 2. Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iacobucci G. Sixty seconds on . . . anosmia. BMJ. 2020;368:m1202. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Lipner SR. Retrospective analysis of smell and taste disturbances associated with dermatological medications reported to the United States Food and Drug Administration and relevance to COVID-19 infections [published online May 20, 2020]. J Am Acad Dermatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abalo-Lojo JM, Pouso-Diz JM, Gonzalez F. Taste and smell dysfunction in COVID-19 patients [published online May 29, 2020]. Ann Otol Rhinol Laryngol. [DOI] [PubMed] [Google Scholar]
- 6. Aggarwal S, Garcia-Telles N, Aggarwal G, et al. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berlin, Germany). 2020;7:91-96. [DOI] [PubMed] [Google Scholar]
- 7. Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study [published online April 23, 2020]. Eur J Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carignan A, Valiquette L, Grenier C, et al. Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study [published online May 29, 2020]. CMAJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coelho DH, Kons ZA, Costanzo RM, et al. Subjective changes in smell and taste during the COVID-19 pandemic: a national survey—preliminary results [published online May 20, 2020]. Otolaryngol Head Neck Surg. [DOI] [PubMed] [Google Scholar]
- 10. Gautier JF, Ravussin Y. A new symptom of COVID-19: loss of taste and smell. Obesity (Silver Spring, Md). 2020;28:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilani S, Roditi R, Naraghi M. COVID-19 and anosmia in Tehran, Iran. Med Hhypotheses. 2020;141:109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hopkins C, Surda P, Whitehead E, et al. Early recovery following new onset anosmia during the COVID-19 pandemic: an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim GU, Kim MJ, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19 [published online May 4, 2020]. Clin Microbiol Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klopfenstein T, Kadiane-Oussou NJ, Toko L, et al. Features of anosmia in COVID-19 [published online April 20, 2020]. Med Malad Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lao WP, Imam SA, Nguyen SA. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS-CoV-2 infection [published online April 22, 2020]. World J Otorhinolaryngol Head Neck Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lechner M, Chandrasekharan D, Jumani K, et al. Anosmia as a presenting symptom of SARS-CoV-2 infection in healthcare workers: a systematic review of the literature, case series, and recommendations for clinical assessment and management [published online May 10, 2020]. Rhinology. [DOI] [PubMed] [Google Scholar]
- 17. Lee Y, Min P, Lee S, et al. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lehrich BM, Goshtasbi K, Raad RA, et al. Aggregate prevalence of chemosensory and sinonasal dysfunction in SARS-CoV-2 and related coronaviruses [published online May 20, 2020]. Otolaryngol Head Neck Surg. [DOI] [PubMed] [Google Scholar]
- 19. Liguori C, Pierantozzi M, Spanetta M, et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection [published online May 18, 2020]. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu JY, Chen TJ, Hwang SJ. Analysis of imported cases of COVID-19 in Taiwan: a nationwide study [published online May 14, 2020]. Int J Environ Res Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China [published online April 11, 2020]. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19 [published online May 13, 2020]. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merza MA, Haleem Al, Mezori AA, Mohammed HM, et al. COVID-19 outbreak in Iraqi Kurdistan: the first report characterizing epidemiological, clinical, laboratory, and radiological findings of the disease. Diabetes Metab Syndrome. 2020;14:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moein ST, Hashemian SMR, Mansourafshar B, et al. Smell dysfunction: a biomarker for COVID-19 [published online April 18, 2020]. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roland LT, Gurrola JG, II, Loftus PA, et al. Smell and taste symptom-based predictive model for COVID-19 diagnosis [published online May 5, 2020]. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Speth MM, Singer-Cornelius T, Obere M, et al. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics [published online May 20, 2020]. Otolaryngol Head Neck Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tong JY, Wong A, Zhu D, et al. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis [published online May 6, 2020]. Otolaryngol Head Neck Surg. [DOI] [PubMed] [Google Scholar]
- 29. Vaira LA, Salzano G, Deiana G, et al. Anosmia and ageusia: common findings in COVID-19 patients [published online April 3, 2020]. Laryngoscope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vetter P, Vu DL, L’Huillier AG, et al. Clinical features of Covid-19. BMJ. 2020;369:m1470. [DOI] [PubMed] [Google Scholar]
- 31. Yan CH, Faraji F, Prajapati DP, et al. Self-reported olfactory loss associates with outpatient clinical course in Covid-19 [published online April 25, 2020]. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zayet S, Klopfenstein T, Mercier J, et al. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients [published online May 16, 2020]. Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hopkins C, Kumar N. Loss of sense of smell as marker of COVID-19 infection. Accessed June 12, 2020 https://www.entuk.org/sites/default/files/files/Loss%20of%20sense%20of%20smell%20as%20marker%20of%20COVID.pdf [DOI] [PMC free article] [PubMed]
- 34. American Academy of Otolaryngology–Head and Neck Surgery. Anosmia, hyposmia, and dysgeusia symptoms of coronavirus disease. Accessed June 12, 2020 https://www.entnet.org/content/aao-hns-anosmia-hyposmia-and-dysgeusia-symptoms-coronavirus-disease
- 35. World Health Organization. Health topic: coronavirus. Accessed June 16, 2020 https://www.who.int/health-topics/coronavirus#tab=tab_3
- 36. Costa KVTd, Carnaúba ATL, Rocha KW, et al. Olfactory and taste disorders in COVID-19: a systematic review [published online June 9, 2020]. Braz J Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsai S-T, Lu M-K, San S, et al. The neurologic manifestations of coronavirus disease 2019 pandemic: a systemic review [published online May 19, 2020]. Front Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moola S, Munn Z, Tufanaru C, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer’s Manual. Joanna Briggs Institute; 2017. [Google Scholar]
- 39. Bénézit F, Le Turnier P, Declerck C, et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19 [published online April 19, 2020]. Lancet Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020;58:295-298. [DOI] [PubMed] [Google Scholar]
- 42. Lechien JR, Cabaraux P, Chiesa-Estomba CM, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients [published online May 22, 2020]. Head Neck. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study [published online April 8, 2020]. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lechien JR, Chiesa-Estomba CM, Hans S, et al. Loss of smell and taste in 2013 European patients with mild to moderate COVID-19 [published online May 26, 2020]. Ann Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Levinson R, Elbaz M, Ben-Ami R, et al. Anosmia and dysgeusia in patients with mild SARS-CoV-2 infection. medRxiv 2020. https://www.medrxiv.org/content/10.1101/2020.04.11.20055483v1 [DOI] [PubMed] [Google Scholar]
- 46. Paderno A, Schreiber A, Grammatica A, et al. Smell and taste alterations in Covid-19: a cross-sectional analysis of different cohorts [published online May 16, 2020]. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vaira LA, Hopkins C, Salzano G, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study [published online May 22, 2020]. Head Neck. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020;71(15):889-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020;42:1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms [published online April 13, 2020]. Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Paderno A, Schreiber A, Grammatica A, et al. Sensorineural smell and taste alterations are highly suggestive of SARS-CoV-2 infection: cross-sectional study in an epidemic area. 2020. 10.2139/ssrn.3576870 [DOI]
- 52. Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806-813. doi:10.1002/alr.22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Agyeman AA, Chin KL, Landersdorfer CB, et al. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(8):1621-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xia S, Liu M, Wang C, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271-280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soler ZM, Yoo F, Schlosser RJ, et al. Correlation of mucus inflammatory proteins and olfaction in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2020;10:343-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Keyhan SO, Fallahi HR, Cheshmi B. Dysosmia and dysgeusia due to the 2019 novel coronavirus: a hypothesis that needs further investigation. Maxillofac Plast Reconstr Surg. 2020;42:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Perlman S, Evans G, Afifi A. Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J Exp Med. 1990;172:1127-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Tables_1_to_5 for Systematic Review and Meta-analysis of Smell and Taste Disorders in COVID-19 by Titus Sunday Ibekwe, Ayotunde James Fasunla and Adebola Emmanuel Orimadegun in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation