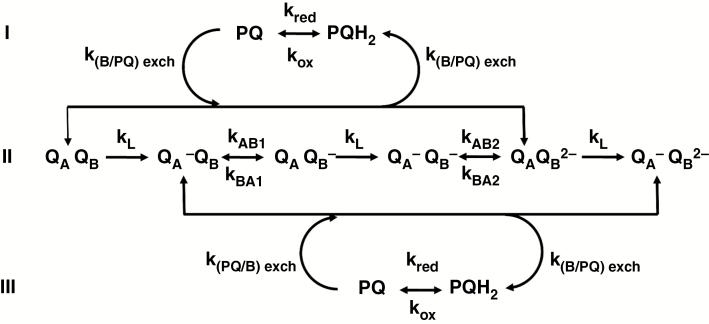
Fig. 5.
Scheme of the two-electron gate (TEG) model and related reactions. The two-electron gate mechanism, by which electrons are transferred from QA to QB, is represented by the reactions on line II. The rate constants are: kL, overall rate constant of QA reduction; kAB1 and kAB2, rate constants of ET from the reduced QA to QB and QB‒, respectively; kBA1 and kBA2, rate constants of backward ET from QB‒ and QB2‒ to QA, respectively. The reactions above and below line II describe the reversible exchange of doubly reduced QB (after its double protonation, which is implicitly assumed) with a PQ molecule from the PQ pool (rate constants k(B/PQ)exch and k(PQ/B)exch); the reversible oxidation of the plastoquinol (rate constants kox and kred) is implicitly assumed to be the result of chlororespiration and cyclical electron flow around PSI. Modified from Lazár and Schansker (2009).