Key Points
Questions
What are the outcomes of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage and excessively high initial systolic blood pressure (≥220 mm Hg)?
Findings
This post hoc analysis of a randomized clinical trial showed higher rates of neurological deterioration and no evidence of reducing hematoma expansion at 24 hours or death or severe disability at 90 days in those who underwent intensive systolic blood pressure reduction.
Meaning
The significantly higher rate of neurological deterioration associated with intensive treatment in patients with initial systolic blood pressure of 220 mm Hg or more warrants caution against broad recommendations for intensive systolic blood pressure reduction in patients with intracerebral hemorrhage.
This secondary analysis of a randomized clinical trial evaluates the differential outcomes of intensive vs standard systolic blood pressure reduction in patients with intracerebral hemorrhage and initial systolic blood pressure of 220 mm Hg or more vs less than 220 mm Hg.
Abstract
Importance
The safety and efficacy of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage who present with systolic blood pressure greater than 220 mm Hg appears to be unknown.
Objective
To evaluate the differential outcomes of intensive (goal, 110-139 mm Hg) vs standard (goal, 140-179 mm Hg) systolic blood pressure reduction in patients with intracerebral hemorrhage and initial systolic blood pressure of 220 mm Hg or more vs less than 220 mm Hg.
Design, Setting, and Participants
This post hoc analysis of the Antihypertensive Treatment of Acute Cerebral Hemorrhage-II trial was performed in November 2019 on data from the multicenter randomized clinical trial, which was conducted between May 2011 to September 2015. Patients with intracerebral hemorrhage and initial systolic blood pressure of 180 mm Hg or more, randomized within 4.5 hours after symptom onset, were included.
Interventions
Intravenous nicardipine infusion titrated to goals.
Main Outcomes and Measures
Neurological deterioration and hematoma expansion within 24 hours and death or severe disability at 90 days, plus kidney adverse events and serious adverse events until day 7 or hospital discharge.
Results
A total of 8532 patients were screened, and 999 individuals (mean [SD] age, 62.0 [13.1] years; 620 men [62.0%]) underwent randomization and had an initial SBP value. Among 228 participants with initial systolic blood pressures of 220 mm Hg or more, the rate of neurological deterioration within 24 hours was higher in those who underwent intensive (vs standard) systolic blood pressure reduction (15.5% vs 6.8%; relative risk, 2.28 [95% CI, 1.03-5.07]; P = .04). The rate of death and severe disability (39.0% vs 38.4%; relative risk, 1.02 [95% CI, 0.73-1.78]; P = .92) was not significantly different between the 2 groups. There was a significantly higher rate of kidney adverse events in participants randomized to intensive systolic blood pressure reduction (13.6% vs 4.2%; relative risk, 3.22 [95% CI, 1.21-8.56]; P = .01), but no difference was observed in the rate of kidney serious adverse events.
Conclusions and Relevance
The higher rate of neurological deterioration within 24 hours associated with intensive treatment in patients with intracerebral hemorrhage and initial systolic blood pressure of 220 mm Hg or more, without any benefit in reducing hematoma expansion at 24 hours or death or severe disability at 90 days, warrants caution against generalization of recommendations for intensive systolic blood pressure reduction.
Introduction
The guidelines1 from the American Heart Association and American Stroke Association for the management of spontaneous intracerebral hemorrhage (ICH) recommend, “For ICH patients presenting with SBP [systolic blood pressure] between 150 and 220 mm Hg and without contraindication to acute blood pressure treatment, acute lowering of SBP to 140 mm Hg is safe (Class I; Level of Evidence A) and can be effective for improving functional outcome (Class IIa; Level of Evidence B)”1(p2040) and “For ICH patients presenting with SBP >220 mm Hg, it may be reasonable to consider aggressive reduction of BP [blood pressure] with a continuous intravenous infusion and frequent BP monitoring (Class IIb; Level of Evidence C).”1(p2040) Class IIb indicates that efficacy is less well established by evidence and additional studies with broad objectives and/or additional registry data will be helpful.
The lower levels of evidence for recommendations for patients with SBP greater than 220 mm Hg are based on the lack of data derived from the Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT2),2 which only included patients with ICH with at least 2 SBP measurements greater than or equal to 150 mm Hg and less than or equal to 220 mm Hg.2,3 The European Stroke Organisation–Karolinska Stroke Update Conference4 has also recommended lowering SBP to less than 140 mm Hg and greater than 110 mm Hg in patients with acute ICH (without recommendations stratified by initial SBP levels) but has recommended avoiding SBP reduction of more than 90 mm Hg to prevent acute kidney injury (grade B; supported by randomized clinical trials and statistical reviews). The guidelines1 admit the lack of existing data regarding SBP reduction in patients with excessively high SBP. We performed a post hoc analysis of the Antihypertensive Treatment of Acute Cerebral Hemorrhage–II (ATACH-II) trial (ClinicalTrials.gov Identifier: NCT01176565) to provide additional data on safety and efficacy of intensive SBP reduction in patients with excessively high initial SBP.
Methods
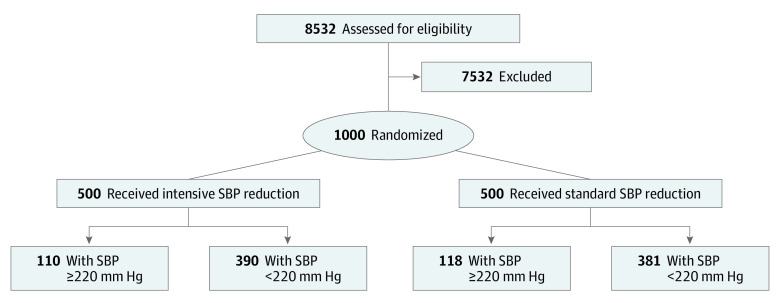
The ATACH-II trial was a multicenter, 2-group, open-label randomized clinical trial to determine the relative efficacy of intensive vs standard SBP reduction initiated within 4.5 hours of symptom onset and continued for the next 24 hours in patients with spontaneous supratentorial ICH. The details are provided in previous publications.5,6,7 The flow diagram is presented in the Figure. In the initial protocol (version 1.0), finalized on January 20, 2010, SBP on admission greater than 180 mm Hg but less than 240 mm Hg on 2 measurements at least 5 minutes apart was used as a key inclusion criterion. A protocol modification (version 3.0-4.0) on September 23, 2011, removed the upper limit (less than 240 mm Hg).8,9,10 At least 1 reading of SBP of 180 mm Hg or more between symptom onset and the initiation of intravenous antihypertensive treatment was required for eligibility.6 The protocol and consent forms were approved by the institutional review board or equivalent ethics committee at each participating site, and all participants or their legally authorized representatives provided written informed consent before randomization. An independent data and safety monitoring board appointed by the National Institute of Neurological Disorders and Stroke monitored the trial.
Figure. Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram.
Data were missing for 1 patient in the standard treatment group. SBP indicates systolic blood pressure.
The first SBP recorded in the emergency department was termed the initial SBP. Treatment could be initiated before randomization to lower the SBP to less than 180 mm Hg, which was consistent with the American Stroke Association Stroke Council guidelines,11 but this was maintained at 140 mm Hg or more before randomization. The SBP immediately prior to randomization was termed the prerandomization SBP, which was different from the initial SBP.
The goal of treatment was to reduce and maintain the hourly minimum SBP in the ranges of 140 to 179 mm Hg in the standard SBP reduction group and 110 to 139 mm Hg in the intensive SBP group throughout the period of 24 hours after randomization. Baseline and 24-hour computed tomography scans were forwarded to the core image analysis center. All adverse events (AEs) were classified with the use of terminology from the Medical Dictionary for Regulatory Activities and systematically reported up to day 7 or hospital discharge, whichever came first. The kidney AEs were grouped as described previously.7 Serious AEs (SAEs) that met the regulatory definition of seriousness were systematically reported up to 3 months after randomization.12 The degree of disability or dependence in daily activities was assessed at 90 days using the modified Rankin scale by an independent investigator unaware of the SBP reduction group. The SAEs were further classified as treatment-associated SAEs occurring within 72 hours after randomization that were considered by the site investigator to be associated with treatment.
The primary outcome was the proportion of participants who experienced death or severe disability (modified Rankin scale score, 4 to 6) at 90 days. Additional outcomes were neurologic deterioration, defined as a decrease from baseline of 2 or more points in Glasgow Coma Scale score or an increase of 4 or more points in the National Institutes of Health Stroke Scale score that was not associated with sedation or hypnotic agent use and was sustained for at least 8 hours within the 24 hours after randomization; hematoma expansion, defined as an increase of 33% or more in the hematoma volume from baseline to 24 hours; and hypotension within 72 hours, defined by a BP that was lower than 90/60 mm Hg.13
Statistical Analysis
We compared demographic features; clinical characteristics and rates of hematoma expansion; neurological deterioration; SAEs, including those ascribed to treatment; serious hypotension; and 90-day death or severe disability between groups with initial SBP of 220 mm Hg or more and less than 220 mm Hg. We subsequently categorized the participants classified with initial SBP of 220 mm Hg or more and those with initial SBP of less than 220 mm Hg according to allocated treatment (intensive or standard SBP reduction) and compared the mentioned variables. We used χ2 tests and analysis of Wilcoxon rank sum tests for categorical and continuous variables. We performed an exploratory analysis comparing the mentioned variables in participants with prerandomization SBP of 220 mm Hg or more and less than 220 mm Hg.
We tested the modifying effect of an initial SBP of 220 mm Hg or more by entering the interaction between treatment group (intensive vs standard SBP reduction) and SBP level (excessively high [≥220 mm Hg] vs <220 mm Hg) using a logistic regression model that included all randomized participants, with 90-day death or severe disability as the primary end point. We also explored the association of race (African American vs others) by entering it as an interaction term with excessively higher SBP in the model. We adjusted for age, Glasgow Coma Scale score, and hematoma volume (all continuous variables). All results were analyzed using SAS Studio Software release 3.8 (Enterprise Edition [SAS Institute]) in November 2019.
Results
Comparison of Participants With Initial SBP of 220 mm Hg or More and Less Than 220 mm Hg
Of a total of 999 participants with data on initial SBP, 228 had initial SBP of 220 mm Hg or more (mean [SD] age, 59.0 [13.2] years; 137 men [60.1%]; Table 1). The mean (SD) age of the group with initial SBP of 220 mm Hg or more was lower than the 771 individuals with initial SBP of less than 220 mm Hg (59.0 [13.2] years vs 62.8 [12.9] years; P < .001). There was a lower proportion of cerebral lobe ICHs in individuals with SBP of 220 mm Hg or more vs less than 220 mm Hg (17 of 228 [7.5%] vs 99 of 771 [12.8%]; P = .03). The mean (SD) minimum SBP measurements at 6 to 7 hours and 23 to 24 hours after randomization were significantly higher among the participants with SBP of 220 mm Hg or more (6-7 hours: 132.2 [17.9] mm Hg vs 128.9 [18.0] mm Hg; P = .01; 23-24 hours: 140.1 [19.0] mm Hg vs 135.8 [17.2] mm Hg; P = .002, respectively). The rate of 90-day death or severe disability was not different between groups (38.7% vs 38.1%; relative risk [RR], 1.02 [95% CI, 0.84-1.23]; P = .87). The rate of hematoma expansion (14.8% vs 25.0%; RR, 0.59 [95% CI, 0.42-0.83]; P = .001) was significantly lower among participants with SBP of 220 mm Hg or more. The neurological deterioration within 24 hours (11.0% vs 9.1%; RR, 1.21 [95% CI, 0.78-1.86]; P = .39) and SAEs (22.4% vs 22.8%; RR, 0.98 [95% CI, 0.74-1.29]; P = .93) were not different between groups. There were no significant differences in the rates of kidney AEs (8.8% vs 5.8%; RR, 1.50 [95% CI, 0.91-2.49]; P = .11) and kidney SAEs (1.3% vs 0.3%; RR, 5.07 [95% CI, 0.85-30.17]; P = .08) between the 2 groups.
Table 1. Demographic and Clinical Characteristics of the Participants According to Initial Systolic Blood Pressure (SBP) Strata.
| Characteristic | Patients, No. (%) | P value | |
|---|---|---|---|
| Initial SBP ≥220 mm Hg (n = 228) | Initial SBP <220 mm Hg (n = 771) | ||
| Age, mean (SD), y | 59.0 (13.2) | 62.8 (12.9) | <.001 |
| Male | 137 (60.1) | 482 (62.5) | .54 |
| Race | |||
| American Indian or Alaska Native | 0 | 4 (0.5) | NA |
| Asian | 110 (48.2) | 451 (58.5) | .006 |
| Black | 43 (18.9) | 88 (11.4) | .003 |
| White | 73 (32.0) | 214 (27.8) | .21 |
| Other or unknown | 2 (0.9) | 14 (1.8) | .55 |
| Ethnicity | |||
| Hispanic | 25 (11.0) | 54 (7.0) | .05 |
| Not Hispanic | 194 (85.1) | 697 (90.4) | .03 |
| Unknown/not reported | 9 (4.0) | 20 (2.6) | .28 |
| Baseline Glasgow Coma Scale score | .21 | ||
| Mean (SD) | 13.5 (2.3) | 13.7 (2.1) | |
| Median (range) | 15 (3-15) | 15 (3-15) | |
| Baseline National Institutes of Health Stroke Scale score | .28 | ||
| Mean (SD) | 12.1 (6.8) | 11.6 (6.9) | |
| Median (range) | 11 (0-39) | 11 (0-40) | |
| Initial SBP, mean (SD), mm Hg | 237.0 (16.0) | 189.8 (19.0) | <.001 |
| SBP prerandomization, mean (SD), mm Hg | 179.6 (29.3) | 173.5 (23.4) | .01 |
| Lowest SBP after randomization, mean (SD), mm Hg | |||
| 2-3 h | 131.3 (19.7) | 129.1 (17.4) | .08 |
| 6-7 h | 132.2 (17.9) | 128.9 (18.0) | .01 |
| 23-24 h | 140.1 (19.0) | 135.8 (17.2) | .002 |
| Intracerebral hematoma volume, mean (SD) | 13.5 (11.8) | 13.8 (12.3) | .90 |
| Location of hemorrhage | |||
| Thalamus | 78 (34.2) | 241 (31.3) | .41 |
| Basal ganglia | 133 (58.3) | 429 (55.6) | .50 |
| Cerebral lobe | 17 (7.5) | 99 (12.8) | .03 |
Abbreviation: NA, not available.
Comparison Between Intensive and Standard SBP Reduction Among Individuals With Initial SBP of 220 mm Hg or More
Of a total of 228 randomized participants with SBP of 220 mm Hg or more, 110 were randomized to intensive SBP reductions. Compared with 118 participants with SBP of 220 mm Hg or more who were randomized to standard SBP reduction, there were no differences in the mean age or proportion of each sex (Table 2). The mean (SD) minimum SBP measurements at 2 to 3 hours, 6 to 7 hours, and 23 to 24 hours after randomization were all significantly lower in the intensive SBP reduction group than the standard SBP reduction group (2-3 hours: 121.7 [15.2] mm Hg vs 140.4 [19.2] mm Hg; P < .001; 6-7 hours: 122.1 [12.2] mm Hg vs 141.7 [17.3] mm Hg; P < .001; 23-24 hours: 127.1 [13.4] mm Hg vs 152.6 [14.8] mm Hg; P < .001). The rates of death or severe disability at 90 days showed no significant difference between groups (39.0% vs 38.4% for intensive and standard SBP reductions, respectively; RR, 1.02 [95% CI, 0.73-1.78]; P = .92; Table 3). Hematoma expansion within 24 hours (13.8% and 15.8% for intensive and standard SBP reductions, respectively; RR, 0.87 [95% CI, 0.46-1.64]; P = .67) was not different between the 2 groups. The rate of neurological deterioration within 24 hours (15.5% vs 6.8%; RR, 2.28 [95% CI, 1.03-5.07]; P = .04) was significantly higher in participants randomized to intensive SBP reduction. The percentage of participants with treatment-associated SAEs within 72 hours after randomization was 2.7% and 0.8% in the intensive and standard SBP reduction groups, respectively (RR, 3.22 [95% CI, 0.34-30.48]; P = .35). The percentage of participants with SAEs during the full study duration was 27.3% and 17.8% in the intensive and standard SBP reduction groups (RR, 1.53 [95% CI, 0.94-2.51]; P = .09). There was a significantly higher rate of kidney AEs in individuals randomized to intensive SBP reduction (13.6% vs 4.2%; RR, 3.22 [95% CI, 1.21-8.56]; P = .01).
Table 2. Demographic and Clinical Characteristics of the Patients According to Treatment Groups Based on Initial Systolic Blood Pressure (SBP) Strata.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| SBP ≥220 mm Hg | SBP <220 mm Hg | |||
| Intensive treatment (n = 110) | Standard treatment (n = 118) | Intensive treatment (n = 390) | Standard treatment (n = 381) | |
| Age, mean (SD), y | 59.9 (13.5) | 58.1 (12.9) | 62.6 (12.9) | 63.1 (12.9) |
| Male | 66 (60.0) | 71 (60.2) | 238 (61.0) | 244 (64.0) |
| Race | ||||
| American Indian or Alaska Native | 0 | 0 | 1 (0.3) | 3 (0.8) |
| Asian | 54 (49.1) | 56 (47.5) | 223 (57.2) | 228 (59.8) |
| Black | 21 (19.1) | 22 (18.6) | 52 (13.3) | 36 (9.4) |
| White | 35 (31.8) | 38 (32.2) | 107 (27.4) | 107 (28.1) |
| Other or unknown | 0 | 2 (1.7) | 7 (1.8) | 7 (1.8) |
| Ethnicity | ||||
| Hispanic | 10 (9.1) | 15 (12.7) | 28 (7.2) | 26 (6.8) |
| Not Hispanic | 96 (87.3) | 98 (83.1) | 351 (90.0) | 346 (90.8) |
| Unknown/not reported | 4 (3.6) | 5 (4.2) | 11 (2.8) | 9 (2.4) |
| Baseline Glasgow Coma Scale score | ||||
| Mean (SD) | 13.4 (2.3) | 13.6 (2.2) | 13.8 (2.0) | 13.7 (2.1) |
| Median (range) | 14 (3-15) | 15 (6-15) | 15 (3-15) | 15 (3-15) |
| Baseline National Institutes of Health Stroke Scale score | ||||
| Mean (SD) | 12.6 (6.9) | 11.6 (6.8) | 11.5 (6.5) | 11.6 (7.3) |
| Median (range) | 12 (1-30) | 10 (0-39) | 11 (0-40) | 11 (0-40) |
| Initial SBP, mean (SD), mm Hg | 236.5 (16.3) | 237.3 (15.8) | 189.7 (19.5) | 190.0 (18.4) |
| SBP prerandomization, mean (SD), mm Hg | 180.2 (30.4) | 178.9 (28.3) | 174.1 (24.0) | 173.0 (22.8) |
| Lowest SBP after randomization, mean (SD), mm Hg | ||||
| 2-3 h | 121.7 (15.2) | 140.4 (19.2) | 120.2 (13.6) | 138.3 (16.0) |
| 6-7 h | 122.1 (12.2) | 141.7 (17.3) | 118.6 (12.8) | 139.4 (16.5) |
| 23-24 h | 127.1 (13.4) | 152.6 (14.8) | 126.5 (12.2) | 145.4 (16.2) |
| Intracerebral hematoma volume, mean (SD) | 13.7 (12.5) | 13.3 (11.2) | 13.4 (11.8) | 14.2 (12.8) |
| Location of hemorrhage | ||||
| Thalamus | 38 (34.5) | 40 (33.9) | 130 (33.3) | 111 (29.1) |
| Basal ganglia | 66 (60.0) | 67 (56.8) | 216 (55.4) | 213 (55.9) |
| Cerebral lobe | 6 (5.5) | 11 (9.3) | 44 (11.3) | 55 (14.4) |
Table 3. Primary, Secondary, and Safety Outcomes in Patients With Initial Systolic Blood Pressures of 220 mm Hg or More, According to Treatment Groups.
| Outcome | Patients, No. (%) | Relative risk (95% CI) | |
|---|---|---|---|
| Intensive treatment (n = 110) | Standard treatment (n = 118) | ||
| Primary outcome (death or severe disability), No./total No. (%)a | 41/105 (39.0) | 43/112 (38.4) | 1.02 (0.73-1.78) |
| Hematoma expansion, No./total No. (%) | 15/109 (13.8) | 18/114 (15.8) | 0.87 (0.46-1.64) |
| Neurologic deterioration within 24 h | 17 (15.5) | 8 (6.8) | 2.28 (1.03-5.07) |
| Treatment-associated serious adverse event within 72 h | 3 (2.7) | 1 (0.8) | 3.22 (0.34-30.48) |
| Any serious adverse event within 3 mo | 30 (27.3) | 21 (17.8) | 1.53 (0.94-2.51) |
| Kidney adverse event within 7 d of discharge | 15 (13.6) | 5 (4.2) | 3.22 (1.21-8.56) |
| Kidney serious adverse event within 7 d of discharge | 2 (1.8) | 1 (0.8) | 2.15 (0.20-23.33) |
Death or disability data at 90 days were available for 217 patients.
Comparison Between Intensive and Standard SBP Reduction Among Participants With Initial SBP Less Than 220 mm Hg
Of a total of 771 randomized participants with initial SBP less than 220 mm Hg, 390 and 381 participants were randomized to intensive and standard SBP reduction, respectively (Table 2). The rate of hematoma expansion within 24 hours was significantly lower in those randomized to intensive SBP reduction than those randomized to standard SBP reduction (20.9% vs 29.2%; RR, 0.72 [95% CI, 0.56-0.92]; P = .009; Table 4). There was a significantly higher rate of kidney AEs in participants randomized to intensive SBP reduction (7.7% vs 3.9%; RR, 1.95 [95% CI, 1.07-3.57]; P = .03).
Table 4. Primary, Secondary, and Safety Outcomes in Patients With Initial Systolic Blood Pressure Less Than 220 mm Hg, According to Treatment Groups.
| Outcome | Patients, No. (%) | Relative risk (95% CI) | |
|---|---|---|---|
| Intensive treatment (n = 390) | Standard treatment (n = 381) | ||
| Primary outcome (death or disability), No./total No. (%)a | 145/376 (38.6) | 138/367 (37.6) | 1.03 (0.85-1.23) |
| Hematoma expansion, No./total No. (%) | 80/382 (20.9) | 108/370 (29.2) | 0.72 (0.56-0.92) |
| Neurologic deterioration within 24 h | 38 (9.7) | 32 (8.4) | 1.16 (0.74-1.82) |
| Treatment-associated serious adverse event within 72 h | 5 (1.3) | 5 (1.3) | 0.98 (0.29-3.35) |
| Any serious adverse event within 3 mo | 98 (25.1) | 78 (20.5) | 1.23 (0.94-1.59) |
| Kidney adverse event within 7 d of discharge | 30 (7.7) | 15 (3.9) | 1.95 (1.07-3.57) |
| Kidney serious adverse event within 7 d of discharge | 2 (0.5) | 0 | NA |
Abbreviation: NA, not available.
Death or disability data at 90 days were available for 743 patients.
Exploratory Analysis of Prerandomization SBP
Of a total of 1000 randomized participants with complete data on prerandomization SBP, 48 had a prerandomization SBP of 220 mm Hg or more. Among those with a prerandomization SBP of 220 mm Hg of more, the rate of death or severe disability at 90 days was significantly higher for individuals randomized to intensive SBP reduction (66.7% vs 29.2%; RR, 2.29 [95% CI, 1.15-4.53]; P = .009). The rate of hematoma expansion (33.3% vs 26.1%; RR, 1.28 [95% CI, 0.52-3.11]; P = .59) was similar between the 2 treatment groups. The rate of neurological deterioration within 24 hours was 20.8% and 4.2% for the intensive and standard SBP reduction treatment groups (RR, 5.00 [95% CI, 0.63-39.67]; P = .19).
Results of Multivariate Analysis and Interaction Test
In the first multivariate model including all randomized participants (n = 960) with data available on modified Rankin scale scores at 90 days, neither intensive SBP reduction nor an initial SBP of 220 mm Hg or more was associated with death or severe disability at 90 days. The interaction between treatment group (intensive vs standard SBP reduction) and initial SBP (≥220 mm Hg vs <220 mm Hg) was not significant. The interaction between race and initial SBP was not significant. In the second analysis, the interaction between treatment groups and prerandomization SBP was significant (SBP ≥220 mm Hg with intensive treatment: RR, 4.8 [95% CI, 1.8-12.7]; SBP ≥220 mm Hg with standard treatment: RR, 0.9 [95% CI, 0.3-2.6]; intensive treatment with SBP ≥220 mm Hg: RR, 4.8 [95% CI, 1.2-18.6]; intensive treatment with SBP <220 mm Hg: RR, 0.9 [95% CI, 0.7-1.3]; P = .02).
Discussion
Intensive SBP reduction was associated with a higher rate of neurological deterioration within 24 hours compared with standard SBP reduction among individuals with initial SBP of 220 mm Hg or more (and this was not seen in those with initial SBP <220 mm Hg). Although hematoma expansion is an important cause of early neurological deterioration,14 we did not identify any difference in the rate of hematoma expansion within 24 hours between participants randomized to intensive vs standard SBP reduction. Another possibility is that intensive SBP reduction resulted in cerebral ischemia among participants with initial SBP of 220 mm Hg or more. A previous study15 found that minimum SBP of 120 mm Hg or less over 72 hours was associated with restricted diffusion lesions (suggestive of ischemia) on magnetic resonance imaging (MRI) in patients with ICH. Garg et al16 found that patients with restricted diffusion lesions on MRI had higher SBP on emergency department admission. However, no differences were identified in SBP changes from intensive care unit admission until MRI completion between patients who did vs did not have restricted diffusion lesions on MRI. Presence of restricted diffusion lesions on MRI was associated with higher rates of death or severe disability at 3 months.
Despite a higher rate of neurological deterioration within 24 hours of randomization in participants with initial SBP of 220 mm Hg or more, the rate of death or severe disability at 90 days was not higher in those randomized to intensive SBP reduction. In an analysis of INTERACT2 that limited inclusion to those with SBP less than 220 mm Hg, participants with higher SBP were at higher risk of early and late neurological deterioration (via an incremental risk for each 10 mm Hg of SBP greater than 150 mm Hg).17 Both early and late neurological deteriorations were associated with death or major disability at 90 days. The goal for individuals with initial SBP of 220 mm Hg or more in ATACH-II was to reduce SBP to less than 180 mm Hg until randomization was performed, leading to stepwise SBP reductions. One possible explanation of lack of association of neurological deterioration with 90-day death or severe disability rates could be that treating physicians may have responded to neurological deterioration by adjustment in the rate of (or avoidance of any further) SBP decline, which could have prevented irreversible ischemia and long-term adverse outcomes. There was a higher rate of kidney AEs but not kidney SAEs in participants randomized to intensive SBP reduction, which may explain the lack of association with 90-day death or severe disability. Intensive SBP reduction was associated with a higher rate of death or severe disability at 90 days in individuals with prerandomization SBP of 220 mm Hg or more, and a significant modifying interaction of prerandomization SBP of 220 mm Hg or more was seen on the association with SBP reduction in an exploratory analysis.
Patients with excessively high initial SBP represent a unique group of patients with ICH. Earlier studies have suggested that this group of patients are at higher risk of hematoma expansion18 and higher mortality.19 The ATACH-II trial was uniquely poised to study patients with excessively high SBP on presentation because of a higher proportion of patients who presented with SBP of 220 mm Hg or more. The mean SBP at presentation in participants in ATACH-II was 200 mm Hg, compared with 170 to 182 mm Hg in INTERACT1,20 INTERACT2,2 the Recombinant Activated Factor VII trial,21 the Factor Seven for Acute Hemorrhagic Stroke trial,22 and the Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial.23 Some previous studies have found a higher rate of hematoma expansion,18 hospital mortality,19 and 28-day mortality24 with very high SBP or mean arterial pressure measured in the initial period of presentation to the hospital. Contrary to such studies,18,19 patients with excessively high initial SBP did not have higher rates of hematoma expansion, death, or disability compared with those with initial SBP less than 220 mm Hg in the ATACH-II trial. Some other studies25,26 have reported that higher rates of hematomata expansion or mortality in patients with ICH and high initial mean arterial pressure or SBP are associated with differences in initial Glasgow Coma Scale scores, hematoma volume, and presence of intraventricular hemorrhage. We did not identify any differences in initial neurological status or hematoma volumes in patients with excessively high initial SBP compared with those with initial SBP less than 220 mm Hg. It is possible that excessively high initial SBP were the result of poorly controlled baseline hypertension and not associated with acute neurological events in some patients.
The American Heart Association and American Stroke Association guidelines in 2015 were based on the results of the INTERACT2 trial.2,3,27 The trial included 2794 patients, of whom 47% had initial SBP of 180 mm Hg or more. The rate of death or dependency at 90 days was slightly higher among patients with initial SBP of 180 mm Hg or more (56%) compared with those with initial SBP less than 180 mm Hg (52%). However, the outcomes associated with intensive SBP reduction did not differ according to initial SBP groups (<180 mm Hg and ≥180 mm Hg) in a prespecified analysis. In the absence of additional data, the guidelines considered it reasonable to aggressively reduce BP with a continuous intravenous infusion and frequent BP monitoring for patients with ICH who present with excessively high SBP (>220 mm Hg), with the clear understanding that usefulness or efficacy is not well established by evidence and based on consensus of opinion. Our data provide additional insight and perhaps evidence for caution and the need for further studies in this unique patient population. It is possible that the modest benefit seen with intensive SBP reduction in the INTERACT2 trial may not be reproducible in patients with initial SBP of 220 mm Hg or more.
Limitations
We acknowledge the limitations of post hoc analysis.28,29,30 Post hoc or subgroup analysis is reserved for the examination of data subsets accrued in the setting of a clinical trial to answer clinically relevant questions that were not addressed in the prespecified data analysis plan. Our analysis can identify a group of patients who may respond differently to treatment than other groups. We acknowledge that the subgroup hypothesis or the direction of outcome was not prespecified.29,30 There was biologic plausibility of different outcomes and perhaps treatment effects in the patients with initial SBP of 220 mm Hg or more as described. The small number of patients with initial SBP of 220 mm Hg or more may not have allowed identification of smaller differences in outcome between the subgroups, such as those based on hematoma location (a type II error).31 Patients with severe neurological injury, high intracranial pressure, or compression of the brainstem were excluded in the ATACH-II study. Therefore, the analysis may not be reflective of patients who have high SBP secondary to intracranial hypertension or brainstem compression.
Conclusion
In the ATACH-II trial, intensive SBP reduction resulted in disproportionately higher rates of neurological deterioration in patients with initial SBP of 220 mm Hg or more and higher rates of death or severe disability at 90 days in patients with prerandomization SBP of 220 mm Hg or more. This post hoc analysis of the ATACH-II data suggests that caution and avoidance of intensive SBP reduction is warranted in patients with initial SBP of 220 mm Hg or more.
References:
- 1.Hemphill JC III, Greenberg SM, Anderson CS, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060. doi: 10.1161/STR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 2.Anderson CS, Heeley E, Huang Y, et al. ; INTERACT2 Investigators . Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355-2365. doi: 10.1056/NEJMoa1214609 [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Palesch YY, Martin R, et al. . Interpretation and Implementation of Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT II). J Vasc Interv Neurol. 2014;7(2):34-40. [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed N, Audebert H, Turc G, et al. . Consensus statements and recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 11-13 November 2018. Eur Stroke J. 2019;4(4):307-317. doi: 10.1177/2396987319863606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15(3):559-576. doi: 10.1007/s12028-011-9538-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qureshi A, Palesch Y, Investigators AI; ATACH II Investigators . Expansion of recruitment time window in antihypertensive treatment of acute cerebral hemorrhage (ATACH) II trial. J Vasc Interv Neurol. 2012;5(suppl):6-9. [PMC free article] [PubMed] [Google Scholar]
- 7.Qureshi AI, Palesch YY, Barsan WG, et al. ; ATACH-2 Trial Investigators and the Neurological Emergency Treatment Trials Network . Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N Engl J Med. 2016;375(11):1033-1043. doi: 10.1056/NEJMoa1603460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beevers G, Lip GY, O’Brien E. ABC of hypertension. Blood pressure measurement. Part I-sphygmomanometry: factors common to all techniques. BMJ. 2001;322(7292):981-985. doi: 10.1136/bmj.322.7292.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien E, Beevers G, Lip GY. ABC of hypertension. Blood pressure measurement. Part III-automated sphygmomanometry: ambulatory blood pressure measurement. BMJ. 2001;322(7294):1110-1114. doi: 10.1136/bmj.322.7294.1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beevers G, Lip GY, O’Brien E. ABC of hypertension: Blood pressure measurement. Part II-conventional sphygmomanometry: technique of auscultatory blood pressure measurement. BMJ. 2001;322(7293):1043-1047. doi: 10.1136/bmj.322.7293.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broderick J, Connolly S, Feldmann E, et al. ; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group . Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update, a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38(6):2001-2023. doi: 10.1161/STROKEAHA.107.183689 [DOI] [PubMed] [Google Scholar]
- 12.Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. JAMA. 1999;281(9):824-829. doi: 10.1001/jama.281.9.824 [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Hashmi MF, Bhattacharya PT Hypotension StatPearls. Updated September 13, 2019. https://www.ncbi.nlm.nih.gov/books/NBK499961/
- 14.Leira R, Dávalos A, Silva Y, et al. ; Stroke Project, Cerebrovascular Diseases Group of the Spanish Neurological Society . Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63(3):461-467. doi: 10.1212/01.WNL.0000133204.81153.AC [DOI] [PubMed] [Google Scholar]
- 15.Buletko AB, Thacker T, Cho SM, et al. . Cerebral ischemia and deterioration with lower blood pressure target in intracerebral hemorrhage. Neurology. 2018;91(11):e1058-e1066. doi: 10.1212/WNL.0000000000006156 [DOI] [PubMed] [Google Scholar]
- 16.Garg RK, Khan J, Dawe RJ, et al. . The influence of diffusion weighted imaging lesions on outcomes in patients with acute spontaneous intracerebral hemorrhage. Neurocrit Care. 2020. doi: 10.1007/s12028-020-00933-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You S, Zheng D, Delcourt C, et al. . Determinants of early versus delayed neurological deterioration in intracerebral hemorrhage. Stroke. 2019;50(6):1409-1414. doi: 10.1161/STROKEAHA.118.024403 [DOI] [PubMed] [Google Scholar]
- 18.Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28(12):2370-2375. doi: 10.1161/01.STR.28.12.2370 [DOI] [PubMed] [Google Scholar]
- 19.Dandapani BK, Suzuki S, Kelley RE, Reyes-Iglesias Y, Duncan RC. Relation between blood pressure and outcome in intracerebral hemorrhage. Stroke. 1995;26(1):21-24. doi: 10.1161/01.STR.26.1.21 [DOI] [PubMed] [Google Scholar]
- 20.Anderson CS, Huang Y, Wang JG, et al. ; INTERACT Investigators . Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7(5):391-399. doi: 10.1016/S1474-4422(08)70069-3 [DOI] [PubMed] [Google Scholar]
- 21.Mayer SA, Brun NC, Begtrup K, et al. ; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators . Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352(8):777-785. doi: 10.1056/NEJMoa042991 [DOI] [PubMed] [Google Scholar]
- 22.Mayer SA, Brun NC, Begtrup K, et al. ; FAST Trial Investigators . Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127-2137. doi: 10.1056/NEJMoa0707534 [DOI] [PubMed] [Google Scholar]
- 23.Butcher KS, Jeerakathil T, Hill M, et al. ; ICH ADAPT Investigators . The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke. 2013;44(3):620-626. doi: 10.1161/STROKEAHA.111.000188 [DOI] [PubMed] [Google Scholar]
- 24.Fogelholm R, Avikainen S, Murros K. Prognostic value and determinants of first-day mean arterial pressure in spontaneous supratentorial intracerebral hemorrhage. Stroke. 1997;28(7):1396-1400. doi: 10.1161/01.STR.28.7.1396 [DOI] [PubMed] [Google Scholar]
- 25.Broderick JP, Diringer MN, Hill MD, et al. ; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators . Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke. 2007;38(3):1072-1075. doi: 10.1161/01.STR.0000258078.35316.30 [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AI, Safdar K, Weil J, et al. . Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage. Stroke. 1995;26(10):1764-1767. doi: 10.1161/01.STR.26.10.1764 [DOI] [PubMed] [Google Scholar]
- 27.Delcourt C, Huang Y, Wang J, et al. ; INTERACT2 Investigators . The second (main) phase of an open, randomised, multicentre study to investigate the effectiveness of an intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT2). Int J Stroke. 2010;5(2):110-116. doi: 10.1111/j.1747-4949.2010.00415.x [DOI] [PubMed] [Google Scholar]
- 28.Hasford J, Bramlage P, Koch G, Lehmacher W, Einhäupl K, Rothwell PM. Inconsistent trial assessments by the National Institute for Health and Clinical Excellence and IQWiG: standards for the performance and interpretation of subgroup analyses are needed. J Clin Epidemiol. 2010;63(12):1298-1304. doi: 10.1016/j.jclinepi.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 29.Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users’ guide to the medical literature. JAMA. 2014;311(4):405-411. doi: 10.1001/jama.2013.285063 [DOI] [PubMed] [Google Scholar]
- 30.Srinivas TR, Ho B, Kang J, Kaplan B. Post hoc analyses: after the facts. Transplantation. 2015;99(1):17-20. doi: 10.1097/TP.0000000000000581 [DOI] [PubMed] [Google Scholar]
- 31.Jennings JM, Sibinga E. Research and statistics: demystifying type I and type II errors. Pediatr Rev. 2010;31(5):209-210. doi: 10.1542/pir.31-5-209 [DOI] [PubMed] [Google Scholar]