Background:
Clinical use of autologous fat for correction of soft-tissue defects in cosmetic and reconstructive procedures has grown in popularity. Graft processing is implicated as one of the variable factors affecting quality, viability, and subsequent graft survival. This study analyzed the in vitro physical and biologic characteristics of lipoaspirate processed using different techniques.
Methods:
Fresh lipoaspirates from patients with informed consent were processed by 4 methods: decantation, centrifugation, the REVOLVE System, and PureGraft. Processed fat grafts were analyzed for yield, composition, tissue particle size and morphology, and viability and function of adipocytes and stem cells. Fat tissue harvested from waste containers of REVOLVE and PureGraft and trapped on REVOLVE paddles was also evaluated.
Results:
Grafts produced by the filtration systems contained the highest percentage of fat tissue, whereas those from decantation contained the lowest percentage, although they have the highest volume yield. In addition, grafts from REVOLVE and PureGraft showed more large-sized particles (>1000 μm) than those from decantation or centrifugation. REVOLVE also preserved significantly higher populations of viable and functional adipocytes and stromal vascular fraction cells when compared with other processing methods. Tissue particles in waste containers of REVOLVE and PureGraft were mostly (>85%) <300 μm and demonstrated a minimal number of viable adipocytes and stem cells. Fat tissues trapped on REVOLVE paddles contained a higher percentage of noninjectable and fibrous collagen bundles.
Conclusion:
Different processing methods result in fat grafts with varying physical and biologic properties, which may contribute to fat graft viability and retention in vivo.
INTRODUCTION
Autologous fat grafting is a widely used technique for soft-tissue filling and augmentation in reconstructive and aesthetic applications, including in the face,1 buttocks, and breasts.2,3 However, optimization is needed to increase engraftment efficiency and achieve more predictable clinical outcomes, particularly improvement of long-term graft volume retention. Reports of volume loss range from 20% to 90% over the first few months,4–8 far from the desirable clinical outcome.
Reasons for inconsistent outcomes following fat grafting procedures are poorly understood. Possible contributing factors include the donor site, harvest procedures, processing methods, graft placement, and recipient site conditions.9 The processing method has been implicated as the most crucial, yet variable, factor affecting clinical outcome, as an appropriate method can ensure graft quality and more consistent results. Lipoaspiration procedures introduce some degree of damage to harvested tissue, leading to generation of contaminants such as free oils, blood cells, collapsed cell debris, stringy tissue, and excessive fluid; if those contaminants are not removed before fat transfer, it can lead to inflammation, tissue necrosis, volume loss, and poor injectability upon transplant.10,11 Processing techniques are therefore designed to achieve removal of contaminants before transfer.
Centrifugation remains the most popular fat processing method, likely owing to ease of use and familiarity. However, there are concerns about the effects of high forces on fat cell viability and efficiency because of the cumbersome nature of processing large volumes of lipoaspirate. Decantation is an alternative method, circumventing force-induced damage to cells, but with a lower overall efficacy.12,13 Recently, filtration-based methods, including the REVOLVE System (LifeCell Corporation, an Allergan Company, Bridgewater, N.J.) and PureGraft (Cytori Therapeutics, Inc, San Diego, Calif.), have become commercially available. These are designed to provide optimal processing of lipoaspirate by preserving the quality and regenerative properties of the native tissue, while removing extraneous fluid and damaged tissue components.11,12
Previous studies have evaluated volume composition of fat grafts processed by REVOLVE and PureGraft.11,12 Both systems were shown to yield a higher and more consistent fat tissue content, with significantly less extraneous fluid, free oil, and red blood cells, than that obtained using decantation and centrifugation. Despite these improvements in graft composition, a clinical study showed a long-term graft retention rate of only 41% with injected fat grafts processed by PureGraft.14 This suggests that factors beyond graft composition, potentially including viability and function of grafted adipocytes and adipose-derived stem cells, may contribute to long-term graft retention.15
This study analyzed the physical and biologic characteristics of fat grafts processed via REVOLVE and PureGraft compared with standard centrifugation and decantation methods. Assessments included graft yield, quality, viability, and function of the processed fat tissue, as well as waste tissue removed by each filtration device.
MATERIALS AND METHODS
Autologous Fat Harvesting
Fresh lipoaspirate samples were collected from 12 healthy consenting donors at local clinics. Donor age ranged from 25 to 66 years (mean, 44 ± 13 years). Tissue samples were obtained from the abdomen, chest, flanks, and back and processed within 2 hours of harvest.
Fat Graft Preparation
Lipoaspirate samples from each donor were allocated for processing by each of the 4 methods, as follows.
Decantation and Centrifugation
An aliquot of lipoaspirate was either set aside and maintained at room temperature for 20 minutes for decantation or centrifuged at 1200g for 3 minutes as previously described16,17 to allow phase separation. Free oil and aqueous layers were carefully aspirated, and the fat tissue layer was retained as the processed graft for further analysis.
REVOLVE and PureGraft
An aliquot of lipoaspirate was loaded into either the REVOLVE System or the PureGraft device and was processed according to the manufacturer’s instructions. The processed fat graft, filtered waste tissue, and tissue from the REVOLVE paddle were collected for further analysis. For all methods, graft yield was measured and graft composition was determined, as previously described.11
Particle Size Analysis
Fat particle size in the processed graft or waste tissue was analyzed using the Horiba Laser Scattering Particle Size Distribution Analyzer (Horiba, Ltd, Kyoto, Japan) according to the manufacturer’s instructions. Briefly, 1–3 mL of tissue samples was loaded into the sample cup. After agitation and circulation in phosphate-buffered saline, particle sizes were measured. Data were plotted to obtain a composite accumulative histogram, showing the undersize distribution of particle populations.
Adipocyte Analysis
Adipocyte Count
Fat graft samples were digested for 1 hour at 37°C with gentle agitation in 200-U/mL collagenase (Sigma-Aldrich, St. Louis, Mo.) at a fat graft:solution ratio of 1:4 (v/v). Fat cells were harvested as described previously18 and stained with a cell viability kit from Nexcelom (Nexcelom Bioscience LLC, Lawrence, Mass.). The number of live and dead cells was counted on a Cellometer K2 (Nexcelom).
Lipolysis Assay
Lipolysis activity in each processed fat graft was measured as described previously.11 All data were expressed as the measured value for each parameter per milliliter of graft/tissue material.
Results of each sample processed by different methods were normalized to the decantation graft and the normalized data (ratio) were compared among different processing methods.
Stromal Vascular Fraction Cell Analysis
Nucleated cells in fat graft samples were isolated as previously described,19–21 and resulting stromal vascular fraction cells were enumerated and used for the following assays.
Fluorescence-activated Cell-sorting Analysis
Harvested stromal vascular fraction cells were washed with phosphate-buffered saline containing 0.5% (weight/volume [w/v]) bovine serum albumin and stained for 30 minutes at 4°C with fluorescein isothiocyanate (FITC)-labeled anti-CD34, phycoerythrin (PE)-labeled anti-CD31, and peridinin-chlorophyll-protein-cyanine 5.5 (PerCP-Cy5.5)-labeled anti-CD45 (BD Biosciences, San Jose, Calif.). The CD45−/CD31−/CD34+ cell population was acquired using a fluorescence-activated cell-sorting Calibur flow cytometer (BD Biosciences) and analyzed with CellQuest software (BD Biosciences). The total number of this population per milliliter of fat graft was calculated.
Colony-forming Unit Culture
Isolated stromal vascular fraction nucleated cells were seeded in a T-25 flask for colony formation as described previously.22 Colony-forming units were counted, and the total number of colony-forming units per milliliter of fat graft was calculated. Ratio of both CD45−/CD31−/CD34+ cell population and colony-forming unit count in each graft from each processing method to decantation was calculated and presented as described above for adipocyte analysis.
Growth Factor Content Measurement
Growth factors in fat grafts processed from one donor sample were extracted with tissue lysis buffer [1:1 (w/v) ratio] and measured with the Bio-Plex Pro Human Cancer Biomarker and Bio-Plex Pro Diabetes Assay kits (BioRad, Hercules, Calif.) according to the manufacturer’s instructions.
Microscopic Evaluations
Macrostructure of Fat Tissue Particles
Tissues were collected from the REVOLVE canister and waste container. The same amount of each sample was dispersed on a glass slide, and images were taken under a Nikon SMZ1000 Stereoscopic Zoom Microscope (Nikon, Melville, N.Y.).
Histology Staining
Tissues were fixed in 10% neutral-buffered formalin, embedded, sectioned, and stained with Masson’s trichrome, as described previously.23 Images were taken with a Nikon Eclipse 80i microscope (Nikon).
Extrusion Force for Injectability of Processed Grafts
Fat grafts were loaded into a 3-mL syringe and expressed through a 21-G needle. The extrusion force (N) required for injection at a rate of 1 mm/s was evaluated on an Instron Model 5865 materials tester (Instron Corporation, Norwood, Mass.). Mean extrusion forces over time for both the REVOLVE fat grafts (n = 3) and paddle-collected tissues (n = 3) were calculated.
Statistical Methods
The paired t test was used to compare the parameters of grafts from the same donor sample processed by any 2 of the 4 different processing techniques. Raw data from all parameters measured in this study were used for each paired t test. Overall statistical significance is defined as a P value of ≤0.05 (2-tailed), and the statistical significance levels for all tests are summarized.
RESULTS
Yield and Composition of Processed Grafts
Decantation produced the highest yield at 47.35% ± 5.65% among the 4 methods (Tables 1 and 2). However, decantation grafts contained the lowest percentage of fat tissue and highest percentage of combined oil and liquid, whereas REVOLVE grafts had the highest percentage of fat tissue and lowest percentage of oil and liquid. PureGraft showed results similar to those of REVOLVE, whereas centrifugation grafts contained the highest oil fraction (Table 1).
Table 1.
Lipoaspirate Processing Yield and Composition of Grafts Processed by Each Method
| Processing Method | Processing Yield (Average ± SEM %) | Composition of Processed Graft (Average ± SEM %) | ||
|---|---|---|---|---|
| Fat Tissue Layer | Oil Layer | Aqueous Infranatant Layer | ||
| Decantation | 47.35 ± 5.65 | 70.10 ± 1.99 | 7.55 ± 1.40 | 22.35 ± 1.11 |
| Centrifugation | 34.71 ± 4.72 | 78.90 ± 3.30 | 9.37 ± 0.98 | 11.73 ± 3.00 |
| REVOLVE | 28.49 ± 2.30 | 88.27 ± 1.47 | 2.10 ± 0.60 | 9.63 ± 1.23 |
| PureGraft | 34.97 ± 4.80 | 84.13 ± 2.59 | 2.85 ± 0.67 | 13.01 ± 2.30 |
SEM, standard error of the mean.
Table 2.
Significance Level of Each Paired t Test (2 Sided)
| P Value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Processing Yield | Composition of | Viable Adipocytes | Lipolysis Activity | CD45−/CD31−/CD34+ | CFU | |||
| Fat | Oil | Liquid | ||||||
| REVOLVE versus decantation | 0.003 | 0.000 | 0.003 | 0.000 | 0.021 | 0.007 | 0.035 | 0.034 |
| REVOLVE versus centrifugation | 0.135 | 0.023 | 0.000 | 0.502 | 0.009 | 0.014 | 0.068 | 0.026 |
| REVOLVE versus PureGraft | 0.084 | 0.106 | 0.191 | 0.158 | 0.026 | 0.037 | 0.077 | 0.042 |
| Decantation versus centrifugation | 0.012 | 0.013 | 0.394 | 0.001 | 0.358 | 0.501 | 0.320 | 0.885 |
| Decantation versus PureGraft | 0.013 | 0.003 | 0.013 | 0.007 | 0.024 | 0.023 | 0.507 | 0.668 |
| Centrifugation versus PureGraft | 0.957 | 0.264 | 0.000 | 0.883 | 0.783 | 0.184 | 0.318 | 0.681 |
CFU, colony-forming unit. Bold numbers indicate statistical significance (P ≤ 0.05).
Fat Tissue Particle Characterization
Approximately 57% of tissue particles in the decantation sample were greater than 1000 μm in size, and approximately 24% of particles were smaller than 300 μm in size (Fig. 1A). Similar results were observed for the centrifugation graft (data not shown). More than 75% of fat tissue particles from REVOLVE and PureGraft grafts were >1000 μm, and only <9% of particles were smaller than 300 μm. In contrast, in both REVOLVE and PureGraft waste containers, most particles (>85%) were smaller than 300 μm. Grafts processed with REVOLVE demonstrated an intact lobule structure with clustered adipocytes and interconnecting mesenchyme (Fig. 1B), whereas tissues in the waste comprised single or small clusters of adipocytes, mainly tissue debris (Fig. 1C).
Fig. 1.
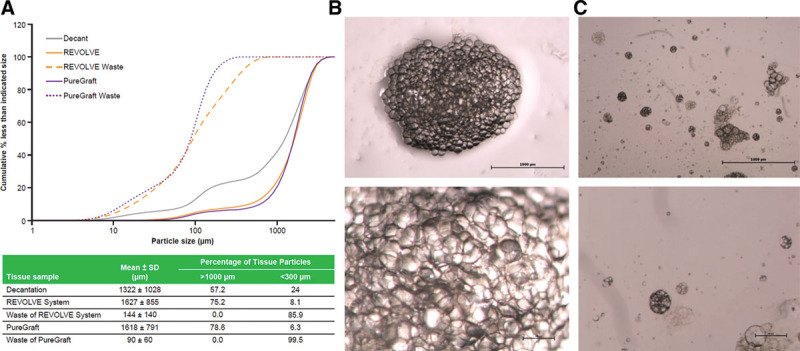
Fat tissue particle analysis. Fat graft was harvested after being processed with either decantation or the 2 filtration systems (REVOLVE System and PureGraft) and analyzed for fat particle size distributions and particle morphology as described in Materials and Methods section. A, Particle size distribution (accumulative frequency) from the different processed grafts indicated (upper). The data shown are from one representative lipoaspirate sample, and the characterization results for each processing method are listed in the table (lower). B and C, Images of fat tissues from the canister (B) and waste container (C) of the REVOLVE System. Upper row: 20×; lower row: 40×.
Viability of the Processed Fat Grafts
REVOLVE grafts showed a statistically higher number of viable adipocytes and significantly higher lipolysis activity (as measured in response to stimulation with a β-adrenergic agonist) than grafts processed by the other 3 methods (Table 2 and Fig. 2). The PureGraft grafts had statistically higher viable adipocytes and lipolysis activity than the decantation grafts, but displayed a similar number of adipocytes as shown by the centrifugation grafts (Table 2 and Fig. 2). Interestingly, the lipolysis activity in centrifugation grafts was not proportionally higher than in decantation grafts, although its processing included a step of concentration by centrifugation.
Fig. 2.
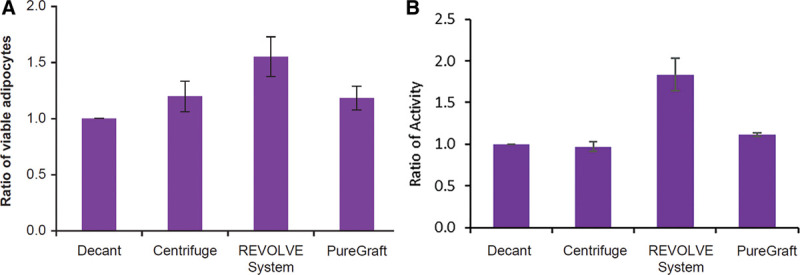
Analysis of adipocytes in each processed graft. A, Ratio of viable adipocytes in grafts processed with centrifugation, REVOLVE System, and PureGraft when compared with decantation. Adipocytes were isolated from the graft samples processed by the 4 methods and analyzed for viable adipocytes as described in Materials and Methods section. The data are presented as average ratio ± SEM. The adipocyte content in the decantation sample, presented as average + SEM, was: 3.763 ± 1.288 × 105 cells/mL graft, N = 5. B, Ratio of adipocyte lipolysis activity. Lipolysis activity was measured in each graft after stimulation with 10-μM isoprenaline as described in Materials and Methods section. The glycerol concentration in the decantation sample after stimulation, presented as average ± SEM, was 38.224 ± 9.292 μg/mL graft, N = 6. SEM indicates standard error of the mean.
Flow cytometry measurements demonstrated that the REVOLVE grafts contained the highest number of CD45−/CD31−/CD34+ cells among the 4 methods but was only statistically higher than the decantation grafts (Table 2 and Fig. 3A). However, the REVOLVE grafts contained a statistically greater number of colony-forming stromal vascular fraction cells (Table 2 and Fig. 3B), indicating that REVOLVE preserved more viable and actively proliferating stromal vascular fraction cells than other methods. Similar to the adipocyte observations, centrifugation grafts showed no difference from decantation grafts in the CD45−/CD31−/CD34+ cell count and even a trend of lower colony-forming unit number, indicating that centrifugation may have damaged some stromal vascular fraction cells.
Fig. 3.
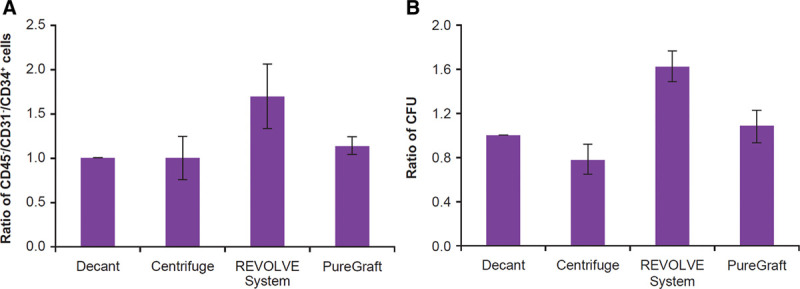
Analysis of SVF cells in each processed graft. SVF cells were isolated from each processed graft and enumerated for specific cell populations by flow cytometry as described in Materials and Methods section. A, The ratio of CD45−/CD31−/CD34+ cells in each processed graft when compared with the decantation graft. The number in the decantation graft was 1.8690 ± 0.7049 × 104 cells/mL graft, average ± SEM, N = 8. B, The ratio of CFU counts in each processed graft when compared with the decantation graft. The average CFU in a decantation graft was 1614 ± 606 CFU/mL graft, average ± SEM, N = 6. CFU indicates colony-forming unit; SEM, standard error of the mean; SVF, stromal vascular fraction.
Growth Factor Content
Owing to extensive washing steps used in REVOLVE and PureGraft processing, those grafts were analyzed for potential loss of growth factors, including basic fibroblast growth factor, hepatocyte growth factor, placental growth factor, vascular endothelial growth factor, insulin-like growth factor–binding protein, and leptin. Grafts from REVOLVE or PureGraft showed a similar level of growth factors as the grafts processed by centrifugation (decantation not included owing to heavy contamination of growth factors in the infranatant phase), indicating that the washing procedures did not reduce growth factors in processed grafts (data not shown).
Tissues Removed during the Filtration Processing
Tissues from waste containers of both filtration systems were harvested and evaluated. Waste tissue from REVOLVE contained approximately 10 times fewer adipocytes (P < 0.031) (Fig. 4A) and up to 60 times fewer colony-forming stromal vascular fraction cells (P < 0.038) (Fig. 4B) than those in fat grafts collected for injection. Similar trends were found for waste tissue from PureGraft (Fig. 4). Furthermore, tissue in the waste of REVOLVE and PureGraft had undetectable lipolysis activity (data not shown).
Fig. 4.
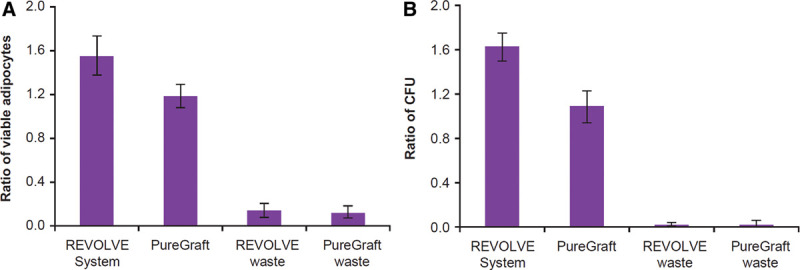
Evaluation of fat tissues harvested from the waste containers of filtration methods. Fat tissues filtered into the waste containers of 2 filtration methods were harvested, and both adipocytes and SVF cells were isolated for analysis as described in Materials and Methods section. A, Ratio of viable adipocytes in fat tissues when compared with the decantation graft (see the fat cell content in decantation sample in Fig. 2). B, Ratio of CFU count when compared with the decantation graft (see the number of CFU in the decantation sample in Fig. 3). CFU indicates colony-forming unit; SVF, stromal vascular fraction.
The REVOLVE system also traps some tissues on its rotating paddle. Histology revealed that those tissues contained a high amount of collagen bundles mingled with broken and ruptured vessel walls (Fig. 5A), whereas processed fat from the collection canister contained intact fat lobules with adipocytes and sporadic intact small vessels embedded in the interconnecting mesenchyme, without thick collagen bundles (Fig. 5B). Furthermore, an injectability test showed that higher forces were needed to extrude the stringy paddle tissue through a 21-G needle compared with the graft tissue (Fig. 6). In addition, the amount of tissue trapped on the paddles was only 6.4% of the total tissue volume in the REVOLVE canister (Table 3), and both viable adipocytes and proliferative stromal vascular fraction cells trapped were only a small fraction of the total number of cells in the REVOLVE canister (Table 3).
Fig. 5.
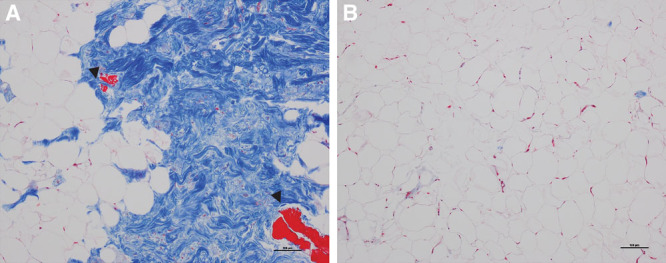
Histology assessment of the tissue harvested from the REVOLVE paddle. A, Tissue harvested from the paddle showed abundant large collagen bundles (large blue area) with some attached adipocyte clusters. B, REVOLVE processed grafts contained clusters of adipocytes surrounded by a thin layer of extracellular matrix without large collagen bundle structures. The collagen bundle–containing tissues exhibited smooth muscle patches from the collapsed blood vessels [arrow heads in (A)]. Masson’s trichrome staining, magnification at 100×.
Fig. 6.
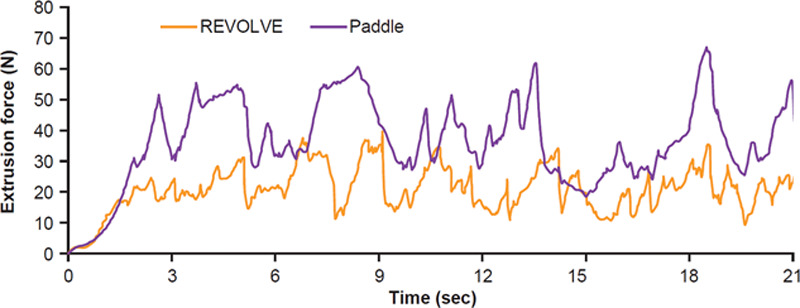
Extrusion force for the paddle-entrapped material in comparison with the nontrapped graft in the REVOLVE canister. Extrusion force of the 2 different materials through a 3-mL syringe and 21-G needle was measured as described in Materials and Methods section. The force was plotted as function of time.
Table 3.
Characterization of Tissue Removed by REVOLVE Paddle
| Samples | Percentage of Total Tissue Volume | Total No. of Viable Cells per 100 mL Tissue | |
|---|---|---|---|
| Viable Adipocytes (×106) | SVF Cells by CFU (×103) | ||
| REVOLVE graft | 93.62 ± 1.59 | 54.7 ± 6.2 | 244.9 ± 19.8 |
| Tissue on paddle | 6.38 ± 1.77 | 1.6 ± 0.5 | 6.7 ± 3.3 |
CFU, colony-forming unit; SVF, stromal vascular fraction.
DISCUSSION
Previous studies showed that contaminants created by liposuction such as cellular debris may lead to inflammation and cell death, which contribute to variable, unpredictable graft retention rate, or even graft failure.24 Although clinical correlation remains to be evaluated between graft fat content and clinical outcomes, a nude rat study12 showed better and more predictable fat tissue retention using grafts with higher fat content. Here we demonstrated that filtration systems produced the highest percentage of fat tissue, consistent with previous studies.11,12 Decantation grafts had the lowest fat tissue percentage, but achieved the highest volume yield due to their high oil, fluid, and tissue debris contaminants. This was supported by the particle size analysis showing decantation grafts had the highest percentage of small particles (<300 μm), whereas grafts processed with REVOLVE and PureGraft contained fewer small particles. Better removal of small particle debris along with free oil and bloody tumescence fluid with REVOLVE and PureGraft is likely due to active washing steps and efficient removal of the liquid phase [drainage across filter membrane by gravity (PureGraft) or constant removal of filtered liquid under vacuum (REVOLVE)].
Native adipose tissue contains various angiogenic growth factors, including vascular endothelial growth factor, hepatocyte growth factor, placental growth factor, angiopoietins, fibroblast growth factor, tumor necrosis factor-α, plasminogen activator inhibitor-1, and metalloproteases to support and maintain vascularization for normal tissue homeostasis.25–28 Extensive washing in a filtration method raises concerns about removing such beneficial growth factors from native fat tissue. In this study, grafts processed with REVOLVE and PureGraft contained a similar level of growth factors as the graft processed by centrifugation, which did not include washing steps. Although our sample size was small, these results were consistent with those of a previous study by Zhu et al.11 Thus, we believe that the washing steps in these 2 filtration systems did not remove growth factors from the processed grafts. This is likely due to the fact that growth factors are fat tissue associated, either tightly bound to the matrix or localized inside the cells. Indeed, Pallua et al29 found that significant quantities of angiogenic growth factors, such as basic fibroblast growth factor and vascular endothelial growth factor, are retained in fat tissue after centrifugation to separate blood components and free oil.
Although the mechanisms of fat graft survival remain poorly understood, there are 2 prevailing theories.15,30 The graft survival theory states that survival depends on the number of viable adipocytes after implantation in vivo, whereas the host replacement theory suggests that survival depends on dynamic remodeling of adipose tissue through activation of adipose-derived stem cells.15 Recently, Kato et al31 further characterized the mechanism of graft survival in vivo and demonstrated that both survival and regenerating processes are present in the newly placed graft. Therefore, in addition to the host conditions at the graft recipient site, a quality fat graft that contains high number of viable adipocytes and proliferative stem cells can maximize graft survival. In the present study, processed fat grafts were further evaluated for the viability and lipolysis activity of adipocytes and proliferative capacity of adipose-derived stem cells by colony-forming unit formation. Interestingly, the grafts from REVOLVE displayed a statistically higher level of viable and active adipocytes than grafts processed by the other 3 methods. Furthermore, grafts processed with REVOLVE also contained the highest number of CD45−/CD31−/CD34+ cells and had significantly higher counts of colony-forming units than grafts processed with other methods. These data strongly indicate the impact of processing methods on graft quality, even between 2 filtration systems. Although both filtration devices have a similar efficiency in removing small tissue particles/debris (Fig. 1), extraneous liquid, and blood components from lipoaspirates as reported in previous studies,11,12 REVOLVE preserves more viable, active, and proliferative cells than PureGraft. The reason for this is unclear but is possibly due to a gentler washing step with the REVOLVE rotating paddle than with the hand massage washing step for PureGraft.
Surprisingly, despite having a significantly higher fat tissue content than decantation grafts in this study and other published studies,12 centrifugation grafts did not show higher lipolysis activity as REVOLVE did, suggesting centrifugation may have caused some damage to adipocyte function. Furthermore, although centrifugation processing resulted in more adipose tissue content than that in the decantation method, the centrifugation graft displayed a similarly low number of progenitor cells as shown by the decantation graft in both CD45−/CD31−/CD34+ cell and colony-forming unit counts, again indicating that the function of these cells in the centrifugation graft was compromised, consistent with previous findings.11 These results also coincide with microscopic observations of altered adipocytes following centrifugation at 1200g for 3 minutes, as previously observed by Condé-Green et al13 using the same conditions as in our study. Several previous in vitro studies concluded that the optimal centrifuge force was 1200g.9,32–36 This speed still affected adipose tissue viability in the present study, although different evaluation methods may account for the varying results. Whether the lower viability of centrifugation graft impacts clinical outcome requires further clinical investigation. In addition, other disadvantages of the centrifugation method include the cumbersome nature of processing large graft volumes and the increased risk of contamination due to the centrifugation procedures.
There is loss of some fat tissues into the waste containers of both the REVOLVE and PureGraft systems, raising concerns for some surgeons, especially when processing lipoaspirates from thinner patients. In this study, fat tissue collected from waste containers of both devices contained only small clusters of fat tissue particles or single cells, which are vulnerable to damage and can easily burst into free oil. Furthermore, tissues from waste containers demonstrated a significantly lower number of viable adipocytes (P < 0.031) (Fig. 4A), as well as undetectable lipolysis activity (data not shown) and significantly lower number of colony-forming stromal vascular fraction cells (P < 0.038) (Fig. 4B), indicating that tissues filtered into waste are true nonviable tissue debris. Removal of this tissue debris is a superior advantage of filtration methods to increase the functionality of a processed graft because the tissue debris was reported to be associated with inflammation and volume loss upon autologous fat grafting.10,11 Furthermore, unlike PureGraft, the REVOLVE device contains rotating paddles within the filter basket. In addition to ensuring thorough but gentle washing of the fat tissue, the paddle was shown to entrap and remove stringy collagen bundle tissue and large pieces of vascular debris while rotating, which may reduce potential clogging of the syringe during graft injection albeit contributing additional small percentage of tissue loss (Table 3). These features make REVOLVE an attractive fat processing method in the operating room.
CONCLUSIONS
Overall, this study demonstrated that different fat processing methods result in fat grafts with varying physical and biologic properties. The variability in fat processing, therefore, may contribute to fat graft viability and retention in vivo. Further studies are needed to correlate these differences with clinical outcomes. Understanding the contribution of various factors in fat processing and their effects may help standardize a clinical protocol for fat grafting in the future.
ACKNOWLEDGMENTS
The authors thank Maryellen Sandor for her contributions in reviewing and editing the manuscript, Eric Stec for data collection, and Ming Luo for assistance in statistical analysis. Editorial assistance was provided to the authors by Katie Dean, PhD, and Susan Sutch, PharmD, of Evidence Scientific Solutions, Inc, Philadelphia, Pa., and funded by Allergan plc. All authors met International Committee of Medical Journal Editors authorship criteria. Neither honoraria nor payments were made for authorship.
Footnotes
Published online 18 August 2020.
These data were presented in part at the 13th Annual International Federation for Adipose Therapeutics and Science (IFATS) Meeting, November 5–8, 2015, New Orleans, LA.
Disclosure: Dr. Fang, Dr. Li, Dr. Huang, Ms. Connell, and Dr. Xu are employees of LifeCell Corporation, an Allergan Company. At the time the research was carried out, Mr. Patel, Ms. Wan, and Mr. Collins were employees of LifeCell Corporation, an Allergan Company.
REFERENCES
- 1.Wetterau M, Szpalski C, Hazen A, et al. Autologous fat grafting and facial reconstruction. J Craniofac Surg. 2012;23:315. [DOI] [PubMed] [Google Scholar]
- 2.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119:775–785; discussion 786. [DOI] [PubMed] [Google Scholar]
- 3.Shapeton AD, Semenovski M, Del Vecchio D. Mega-volume fat transplantation to the breast and buttocks: a new surgical technique that brings new anesthetic challenges. Open J Anesthesiol. 2014;5:27–32 [Google Scholar]
- 4.Kaufman MR, Miller TA, Huang C, et al. Autologous fat transfer for facial recontouring: is there science behind the art? Plast Reconstr Surg. 2007;119:2287–2296 [DOI] [PubMed] [Google Scholar]
- 5.Chajchir A. Fat injection: long-term follow-up. Aesthetic Plast Surg. 1996;20:291–296 [DOI] [PubMed] [Google Scholar]
- 6.Niechajev I, Sevćuk O. Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg. 1994;94:496–506 [DOI] [PubMed] [Google Scholar]
- 7.Ersek RA. Transplantation of purified autologous fat: a 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87:219–227; discussion 228 [PubMed] [Google Scholar]
- 8.Lewis CM. The current status of autologous fat grafting. Aesthetic Plast Surg. 1993;17:109–112 [DOI] [PubMed] [Google Scholar]
- 9.Khater R, Atanassova P. Agullo F. Autologous fat grafting—factors of influence on the therapeutic results, current concepts in plastic surgery. In: Current Concepts in Plastic Surgery. 2012;London, UK: InTech; 183–210 [Google Scholar]
- 10.Shiffman MA. Shiffman MA. History of autologous fat transplant survival. In: Autologous Fat Transfer: Art, Science, and Clinical Practice. 2010;Heidelberg, Germany:Springer-Verlag Berlin Heidelberg; 5–10 [Google Scholar]
- 11.Zhu M, Cohen SR, Hicok KC, et al. Comparison of three different fat graft preparation methods: gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast Reconstr Surg. 2013;131:873–880 [DOI] [PubMed] [Google Scholar]
- 12.Ansorge H, Garza JR, McCormack MC, et al. Autologous fat processing via the REVOLVE system: quality and quantity of fat retention evaluated in an animal model. Aesthet Surg J 2014;34:438–447 [DOI] [PubMed] [Google Scholar]
- 13.Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63:1375–1381 [DOI] [PubMed] [Google Scholar]
- 14.Gerth DJ, King B, Rabach L, et al. Long-term volumetric retention of autologous fat grafting processed with closed-membrane filtration. Aesthet Surg J 2014;34:985–994 [DOI] [PubMed] [Google Scholar]
- 15.Bellini E, Grieco MP, Raposio E. The science behind autologous fat grafting. Ann Med Surg (Lond) 2017;24:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman SR. Lipoinfiltration of the upper lip white roll. Aesthet Surg J 1994;14:231–234 [Google Scholar]
- 17.Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001;28:111–119 [PubMed] [Google Scholar]
- 18.Angle BM, Do RP, Ponzi D, et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 2013;42:256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mailey B, Hosseini A, Baker J, et al. Adipose-derived stem cells: methods for isolation and applications for clinical use. Methods Mol Biol. 2014;1210:161–181 [DOI] [PubMed] [Google Scholar]
- 20.Raposio E, Simonacci F, Perrotta RE. Adipose-derived stem cells: comparison between two methods of isolation for clinical applications. Ann Med Surg (Lond) 2017;20:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raposio E, Bertozzi N. Isolation of ready-to-use adipose-derived stem cell (ASC) pellet for clinical applications and a comparative overview of alternate methods for ASC isolation. Curr Protoc Stem Cell Biol. 2017;41:1F.17.1–1F.17.12 [DOI] [PubMed] [Google Scholar]
- 22.Staszkiewicz J, Frazier TP, Rowan BG, et al. Cell growth characteristics, differentiation frequency, and immunophenotype of adult ear mesenchymal stem cells. Stem Cells Dev. 2010;19:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 2008;Edinburgh, Scotland: Churchill Livingstone; [Google Scholar]
- 24.Girard AC, Mirbeau S, Gence L, et al. Effect of washes and centrifugation on the efficacy of lipofilling with or without local anesthetic. Plast Reconstr Surg Glob Open. 2015;3:e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukumura D, Ushiyama A, Duda DG, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–e97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouloumié A, Lolmède K, Sengenès C, et al. Angiogenesis in adipose tissue. Ann Endocrinol (Paris) 2002;63:(2 Pt 1)91–95 [PubMed] [Google Scholar]
- 27.Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. Faseb J 2004;18:983–985 [DOI] [PubMed] [Google Scholar]
- 28.Lafontan M. Fat cells: afferent and efferent messages define new approaches to treat obesity. Annu Rev Pharmacol Toxicol. 2005;45:119–146 [DOI] [PubMed] [Google Scholar]
- 29.Pallua N, Pulsfort AK, Suschek C, et al. Content of the growth factors bFGF, IGF-1, VEGF, and PDGF-BB in freshly harvested lipoaspirate after centrifugation and incubation. Plast Reconstr Surg. 2009;123:826–833 [DOI] [PubMed] [Google Scholar]
- 30.Peer LA. Cell survival theory versus replacement theory. Plast Reconstr Surg (1946) 1955;16:161–168 [DOI] [PubMed] [Google Scholar]
- 31.Kato H, Mineda K, Eto H, et al. Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg. 2014;133:303e–313e [DOI] [PubMed] [Google Scholar]
- 32.Rohrich RJ, Sorokin ES, Brown SA. In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site. Plast Reconstr Surg. 2004;113:391–395; discussion 396–397. [DOI] [PubMed] [Google Scholar]
- 33.Ramon Y, Shoshani O, Peled IJ, et al. Enhancing the take of injected adipose tissue by a simple method for concentrating fat cells. Plast Reconstr Surg. 2005;115:197–201; discussion 202 [PubMed] [Google Scholar]
- 34.Xie Y, Zheng D, Li Q, et al. The effect of centrifugation on viability of fat grafts: an evaluation with the glucose transport test. J Plast Reconstr Aesthet Surg. 2010;63:482–487 [DOI] [PubMed] [Google Scholar]
- 35.Kim IH, Yang JD, Lee DG, et al. Evaluation of centrifugation technique and effect of epinephrine on fat cell viability in autologous fat injection. Aesthet Surg J 2009;29:35–39 [DOI] [PubMed] [Google Scholar]
- 36.Minn KW, Min KH, Chang H, et al. Effects of fat preparation methods on the viabilities of autologous fat grafts. Aesthetic Plast Surg. 2010;34:626–631 [DOI] [PubMed] [Google Scholar]


