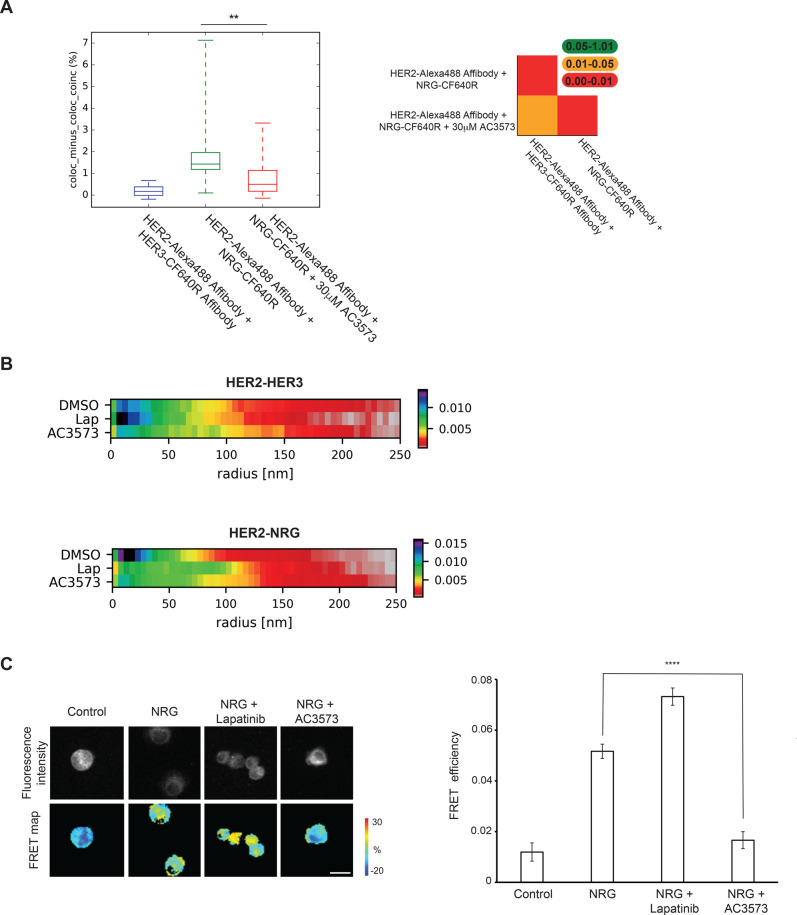
Figure 5. AC3573 compound abrogates the formation of the active HER2–HER3 heterodimer.
(A) Left: two-colour single particle tracking (SPT) on live CHO cells showing the fraction of tracks where two HER2 and HER3 molecules labelled with Alexa 488-Affibody and CF640R SE-Affibody or NRG-CF640R, respectively, spent at least five 50 ms frames together within <1 pixel (pairwise particle colocalisation fraction) for cells treated or not with 30 μM AC3573. Right: significance of differences between compared conditions in two-colour SPT. The plots show tables from the two-tailed Kolmogorov–Smirnov test P-value. (B) Heat map of the probability of the distance of nearest HER2 neighbour of HER3. Cluster measurements from STORM data taken from SK-BR-3 cells labelled with HER2-Alexa488 Affibody and HER3-CF640R SE Affibody (HER2–HER3) or NRG-CF640R SE (HER2-NRG) ± 1 μM lapatinib or 30 μM AC3573 compound. (C) AC3573 blocks NRG-induced dimerisation of HER2 and HER3 receptors in SK-BR-3 breast cancer cells. Cells were seeded on glass coverslips and 72 h later serum-starved for additional 24 h before treatment with AC3573 (30 μM) or lapatinib (1 μM) for 1 h. NRG was added at 10 nM for 15 min. Cells were fixed and stained with anti-HER2-Cy5 and anti-HER3-Alexa-fluor-546 and fluorescence lifetime images were acquired on an open-microscope system. The inhibitory effect of AC3573 is statistically significant (NRG vs NRG + AC3573) with P < 0.0001. The difference between untreated (control) and NRG or lapatinib-treated cells is statistically significant too (P < 0.0001). Scale bar is 25 μm.