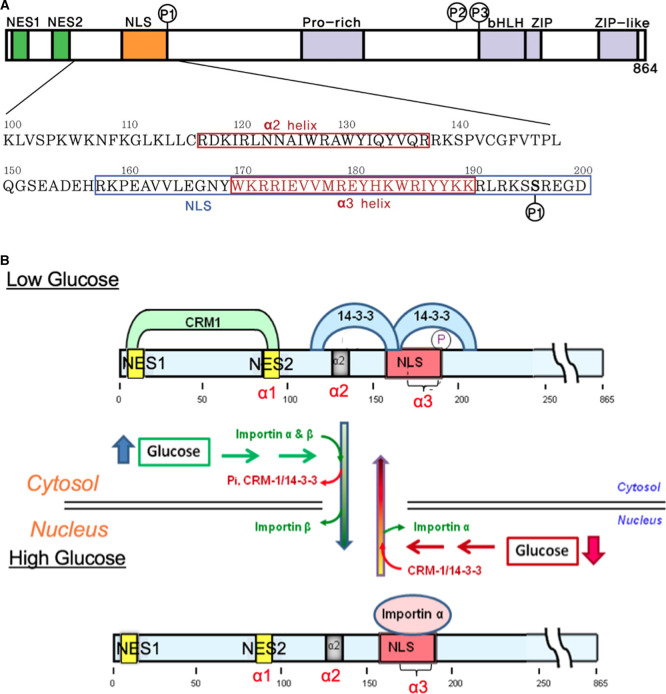
Figure 1. ChREBP domain organization including phosphorylation sites and subcellular localization under low and high glucose concentrations.
(A) Domain organization and the sequences for the N-terminal glucose-responsive regulatory region of ChREBP. The ChREBP function of residues 100–200 in the N-terminal regulatory region, including the α2, and α3 helices, and the NLS (nuclear localization signal) site are depicted. (B) Proposed model of the regulation of ChREBP subcellular localization by 14-3-3 and importin α binding in response to glucose. In response to high glucose, phosphorylated (inactive) ChREBP, complexed with 14-3-3 in the cytosol, is first dephosphorylated and releases 14-3-3 and CRM1. Dephosphorylated (active) ChREBP forms a complex with importin α and β and is translocated into the nucleus. It is possible that 14-3-3 may remain bound to ChREBP during import. A decrease in glucose results in inactivation of ChREBP by phosphorylation by PKA that occurs within the nucleus and complex formation with 14-3-3 and CRM1 followed by translocation to the cytosol. Note that for clarity, 14-3-3 proteins (27 kD) and importin α are not presented to scale. Modified from ref. [5].