Figure 3.
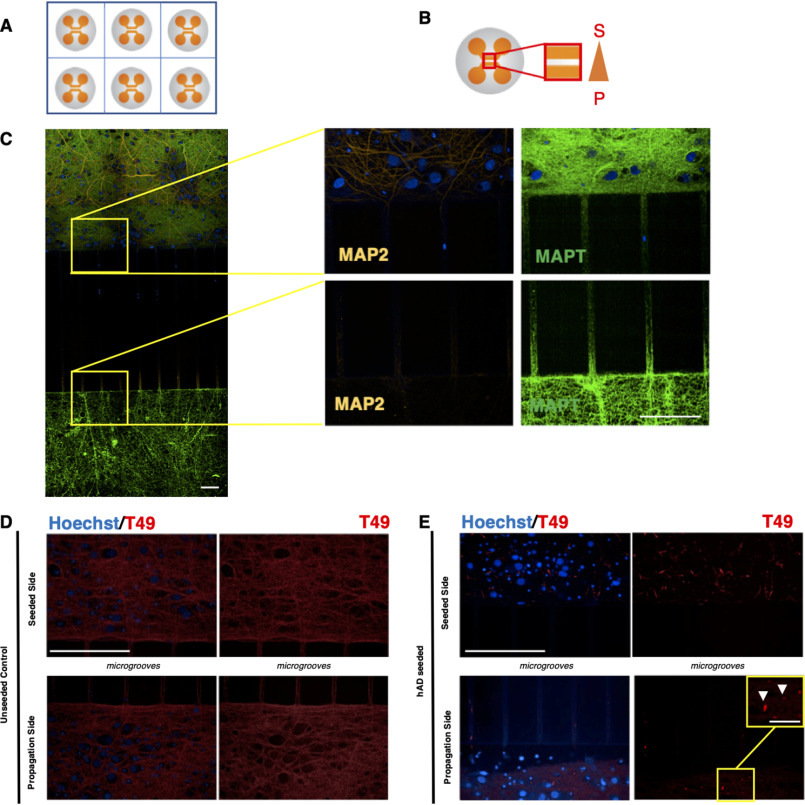
RCNs were cultured in two-compartment microfluidic devices in a 6-well plate assay format to enable the study of trans-neuronal aggregated Tau. A, schematic representation of a typical 6-well plate microfluidic assay format. B, the area of biological interest in a typical two-compartment microfluidic device is highlighted within the red box. To achieve fluidic isolation, the propagation side (bottom chamber, P) received almost 2-fold higher medium volume compared with the seeded side (top chamber, S). C, immunocytochemistry demonstrated that for RCNs cultured for 3 weeks in two-compartment microfluidic devices, only the axons (MAPT) can grow through the 450-μm-long microgrooves rather than the dendrites (MAP2). Figure bar, 15 μm; zoomed image bar, 50 μm. RCNs cultured in two-compartment microfluidic devices template the hAD seed and propagate. D and E, the control unseeded neurons represent a diffused T49 staining (D), whereas in the presence of the hAD seed (E), the neurons demonstrate neuritic thread-like inclusion morphology (white arrows). The results have been reproduced in two independent experiments. Bar, 50 μm. Image acquisition with Opera Phenix and 40× objective. Zoomed images are indicated by yellow squares. Bar, 12.5 μm.