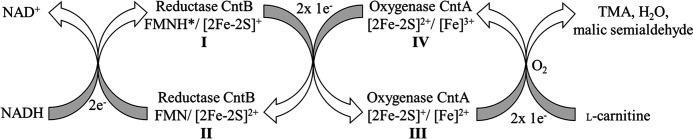
Figure 10.
Proposed redox catalytic cycle of carnitine monooxygenase. A redox catalytic cycle for carnitine monooxygenase was proposed based on the experimentally characterized redox states of the present study. Reduction of CntB by the two-electron donor NADH results in the FMNH*/[2Fe-2S]+ state (I). Subsequent two-electron transfer onto CntA reveals the electron donor in the CntB FMN/[2Fe-2S]2+ state (II) and activates the electron acceptor in the CntA [2Fe-2S]+/[Fe]2+ (III). Subsequent electron transfer onto O2 results in the CntA [2Fe-2S]2+/[Fe]2+ redox state, which resembles the as-purified state of this protein (IV) (compare to Fig. 7B). The reduction of O2 then leads to the formation of the monooxygenated l-carnitine product (not shown) (compare to Fig. 2A) and H2O. Cleavage of the C–N bond of the intermediate then results in the formation of malic semialdehyde and TMA.