Figure 9.
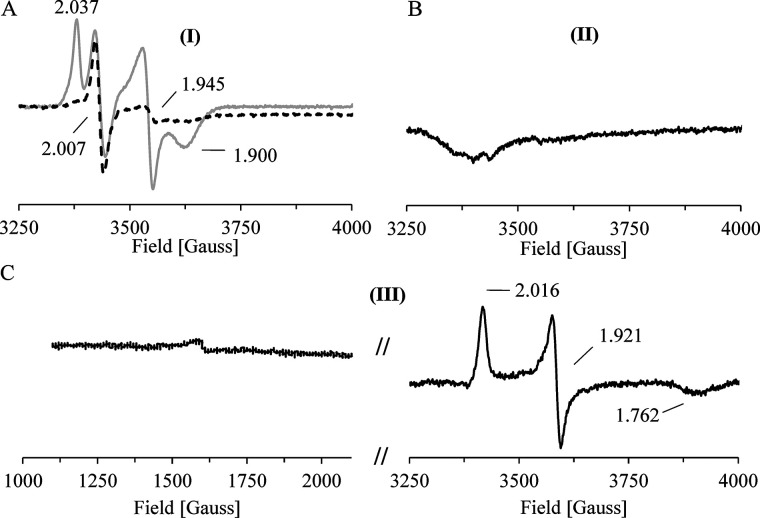
EPR analysis of single-turnover experiments. A–C, intermediate states of the redox catalysis of CntB and CntA were spectroscopically characterized. All experiments performed under strict anaerobic conditions. A, an immobilized CntB sample was incubated in the presence of 2 mm NADH, the cofactor was removed by extensive washing, and the eluted protein was subjected to low-temperature EPR measurements at 15 K. A [2Fe-2S]+ cluster signal (g1 = 2.037, g2 = 1.945, and g3 = 1.900) and a semiquinone signal (g = 2.007) was observed (gray trace). Identical measurements at higher temperature (85 K) revealed the specific depletion of the g = 1.945 [2Fe-2S]+ cluster signal (black dashed line). A CntB FMNH*/[2Fe-2S]+ species (I) was proposed and was subsequently used in a single-turnover experiment. B, in the presence of CntA, a two-electron transfer from (I) resulted in the EPR-silent state CntB FMN [2Fe-2S]2+ (II) (after CntA removal and CntB elution). C, EPR spectroscopic characterization of the resulting CntA revealed the transfer of two electrons. A Rieske [2Fe-2S]+/[Fe]2+ state was concluded (III).