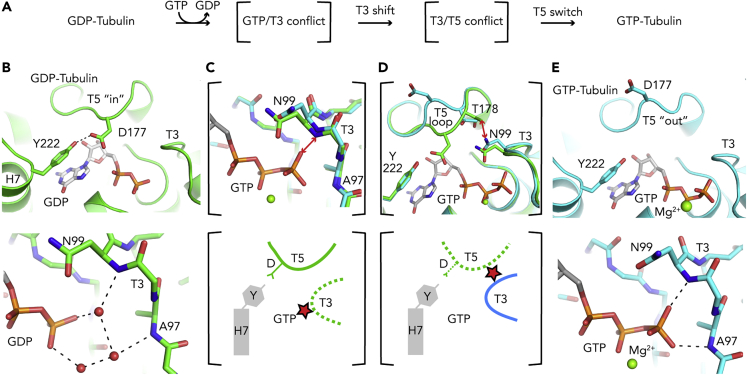
Figure 3.
How GTP Binding Activates Tubulin for Microtubule Assembly
(A) A cascade of rearrangements occurs in the β-tubulin nucleotide binding site upon GTP binding.
(B) With bound GDP, the T5 loop adopting an “in” conformation is prone to interact with the H7 helix Tyr222 residue (Top), and the interaction of GDP with the T3 loop is mediated by water molecules (red spheres) (bottom).
(C) Following GTP binding, GTP would come too close to the NH amide of Asn99 from T3 (2.2 Å distance), illustrated by a double red arrow in the superposed structures of GDP- and GTP-tubulin (top; GDP is not shown) and by a red star in the schematic drawing (bottom).
(D) The conformation of T3 in GTP-tubulin is not compatible with the T5 “in” structure, as it would lead to a steric conflict between Asn99 and Thr178 (double red arrow and red star in the top and bottom panels, respectively).
(E) In GTP-tubulin, T5 has switched to an “out” conformation (top) and T3 interacts directly with the GTP γ-phosphate (bottom). GDP-tubulin is in green and GTP-tubulin in cyan. Selected hydrogen bonds are shown as dashed black lines in (B) and (E).