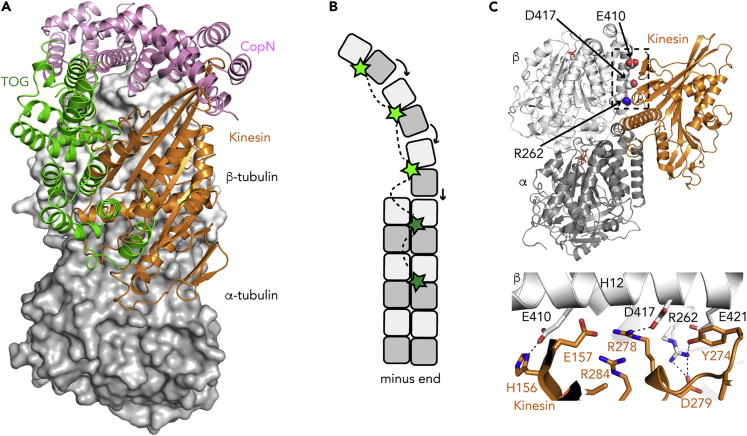
Figure 5.
The Mechanism of MAPs that Bind to the Microtubule, as Deduced from Their Complexes with Tubulin
(A) Composite figure of the structure of tubulin bound to a TOG domain and to a kinesin motor domain. CopN is shown as a reference.
(B) A model for the microtubule polymerase activity of the TOG domain-containing XMAP215 protein. TOGs 1–3 (bright green) capture soluble tubulin and favor longitudinal contacts (arrows), whereas TOG4 and 5 (dark green) and the C-terminal domain (not shown) anchor XMAP215 at the microtubule plus end, possibly enhancing inter-protofilament interactions.
(C) Structural basis for the effect of disease-related tubulin mutations on the interaction with kinesins. (Top) Overview of the structure of tubulin bound to kinesin-1. β-Tubulin residues whose mutation is found in CFEOM3 are highlighted. (Bottom) Close-up of the part framed in the top panel. Selected interactions, involving tubulin residues mutated in CFEOM3, are shown as dashed lines.