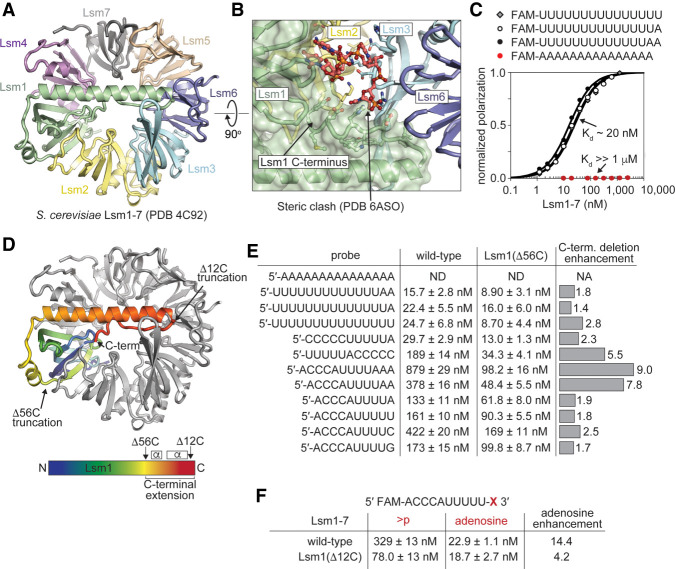
FIGURE 3.
The elongated carboxy-terminal region of Lsm1 in the S. pombe Lsm1–7 ring attenuates RNA binding. (A) Structure of the S. cerevisiae Lsm1–7 ring in the absence of bound RNA. The carboxy-terminal region of Lsm1 crosses the distal face of the ring, occluding the central pore. (B) Superposition of U6 snRNA from the homologous S. cerevisiae Lsm2–8 ring with the S. cerevisiae Lsm1–7 ring. The alignment in this figure was achieved by superposition of Lsm2, Lsm3, and Lsm6 components of the rings, which share a pairwise r.m.s.d of 0.5 Å. A steric clash is visible between the 3′ uridine phosphate and the carboxy-terminal region of Lsm1. (C) In vitro fluorescence polarization binding assays show that S. pombe Lsm1–7 tightly binds to polyuridylate tracts without accessory proteins, like Pat1. In contrast, S. pombe Lsm1–7 lacks detectable affinity for polyadenylate. (D) Designed truncations of the Lsm1 carboxyl terminus. Lsm1 is colored blue to red from the amino- and carboxyl-termini, respectively, and regions selected for truncation are annotated in the figure, resulting in truncation of the last 12 residues that fold back into the pore, or truncation of the entire helical region that spans the distal face of the ring. (E) Binding assays showing that deletion of the Lsm1 carboxy-terminal region generally enhances binding affinity for RNAs that harbor polyuridine tracts, and the relative enhancement is greatest for the weakest binding RNAs. (F) A strong preference for an adenosine 3′ terminus over a uridine cyclic phosphate 3′ terminus is diminished upon deletion of the carboxy-terminal 12 residues of Lsm1.