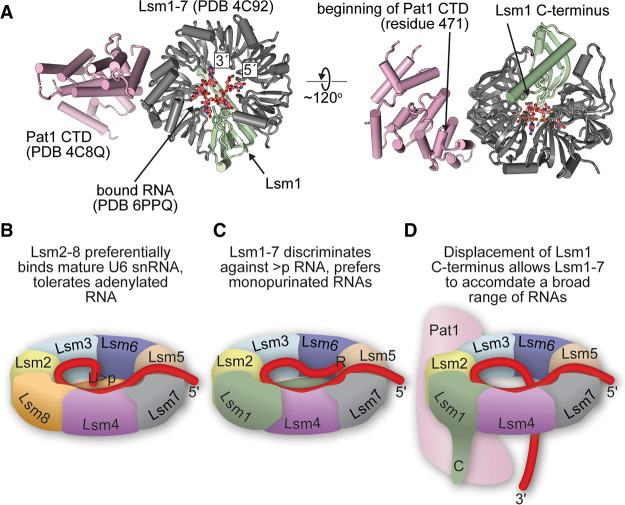
FIGURE 6.
Proposed model for Pat1-regulated gating of the Lsm1–7 RNA-binding pore. (A) Relative orientations of RNA, Lsm1–7 and Pat1 proteins, derived from structures of S. pombe Lsm1–7 with RNA and S. cerevisiae Lsm1–7 with the carboxy-terminal domain of Pat1 (Sharif and Conti 2013). (B) Model for RNA binding specificity in Lsm rings. The Lsm2–8 ring contains a uridine-cyclic phosphate binding site that includes the carboxyl terminus of Lsm8 and endows specificity for Usb1 processed U6 snRNA. (C) In contrast, the carboxy-terminal region of Lsm1 in the Lsm1–7 ring antagonizes association of the ring with uridine-cyclic phosphate terminated RNA, while allowing association of the ring with 3′ monopurinated polyuridylate tracts. (D) Deletion of the carboxy-terminal region of Lsm1 allows the ring to associate with a broader range of RNAs. In vivo, the role of Lsm1 truncation may be mimicked by displacement of the carboxyl terminus by association of the ring with the Pat1 cofactor. It remains to be seen if RNA is capable of threading all the way through the pore of the RNA, as depicted here, or rather enters and exits the pore on the proximal face alone.