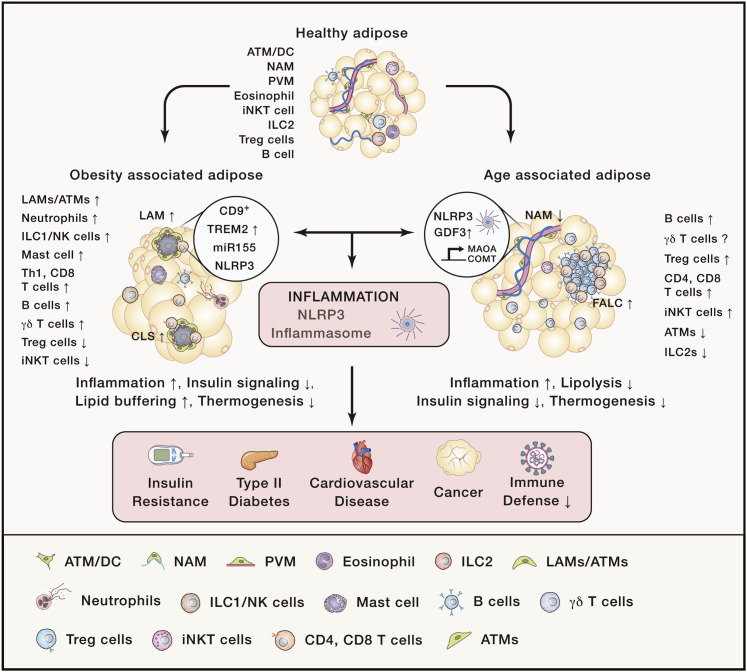
Figure 2.
Obesity and Aging Lead to Distinct Changes in the Immune Profile of Adipose Tissue to Drive Inflammation and Pathology
Healthy, lean adipose tissue is associated with anti-inflammatory immune subsets, such as CD206-expressing macrophage subsets (ATMs, NAMs, and PVMs), IL-4-expressing eosinophils, IL-10-expressing iNKT cells, B cells, Treg cells, and IL-5-expressing ILC2s. Energy imbalance leads to a disruption of immune and metabolic homeostasis, leading to an infiltration of inflammatory immune cells such as IL-6- and TNF-α-producing LAMs, elastase-producing neutrophils, IgG2C-producing B cells, degranulating mast cells, IFN-γ-producing CD4 T cells, IL-17-producing γδ T cells, and cytotoxic CD8 T cells. Accumulation of danger-associated molecular patterns (DAMPs) such as free fatty acids (FAs), oxidized LDL (oxLDL), cholesterol crystals, and islet amyloid polypeptides (IAPP) in obesity further drives the activation of NLRP3 inflammasomes. These changes in immune-mediated inflammation drive pathologies such as insulin resistance, type II diabetes, cardiovascular disease, and cancer while weakening immune defense against pathogens, leading to higher risk for premature death. Unlike obesity, which is characterized by an accumulation CLSs, where immune cells such as T cells and CD9+ macrophages associated with increased lipid processing and inflammation accumulate to deal with dying adipocytes, aging causes a reduction of adipose tissue macrophages that increase GDF3 and catecholamine degradation enzymes such as MAOA and COMT to promote lipolysis resistance. Aging also leads to an increase of FALCs, where B cells and T cells accumulate and contribute to dysregulated adipose homeostasis such as reduced insulin signaling and thermogenesis. Although γδ T cells increase with obesity, iNKT cells and Treg cells increase with age and decrease with obesity, and although no marked changes in eosinophils are reported in aged adipose, obesity is associated with significant decreases in eosinophil populations. These differences in immune profiles and immune lymphoid structures during aging and obesity suggest that distinct mechanisms contribute to the tissue dysfunction and pathologies associated with each condition.