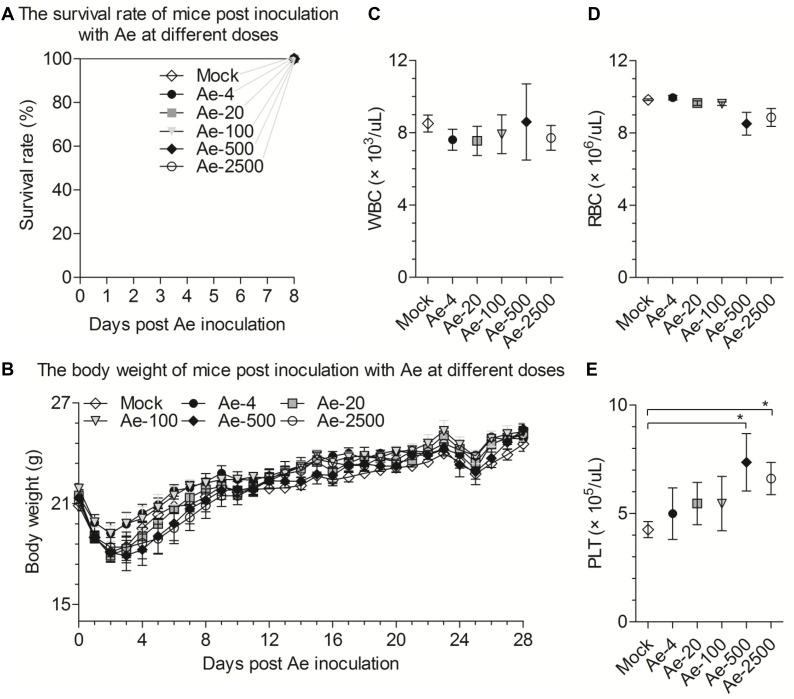
Fig. 5.
The toxicity study of Ae in vivo. (A) Survival curves of mice were orally administered Ae in a single dose of 0, 4, 20, 100, 500, or 2500 mg/kg bw in 8 days. Subchronic toxicity evaluation of oral Ae with a single dose of 0, 4, 20, 100, 500, or 2500 mg/kg bw in mice. (B) The oral doses of Ae for the 6 groups of mice were 0, 4, 20, 100, 500, or 2500 mg/kg bw, respectively. Body weight was monitored and plotted. Mice were sacrificed at day 28, and white blood cells (WBC) (C), red blood cells (RBC) (D), platelets (PLT) (E) were detected. Data are represented as mean ± SD, n = 6. *stands for p < 0.05.