Figure 2.
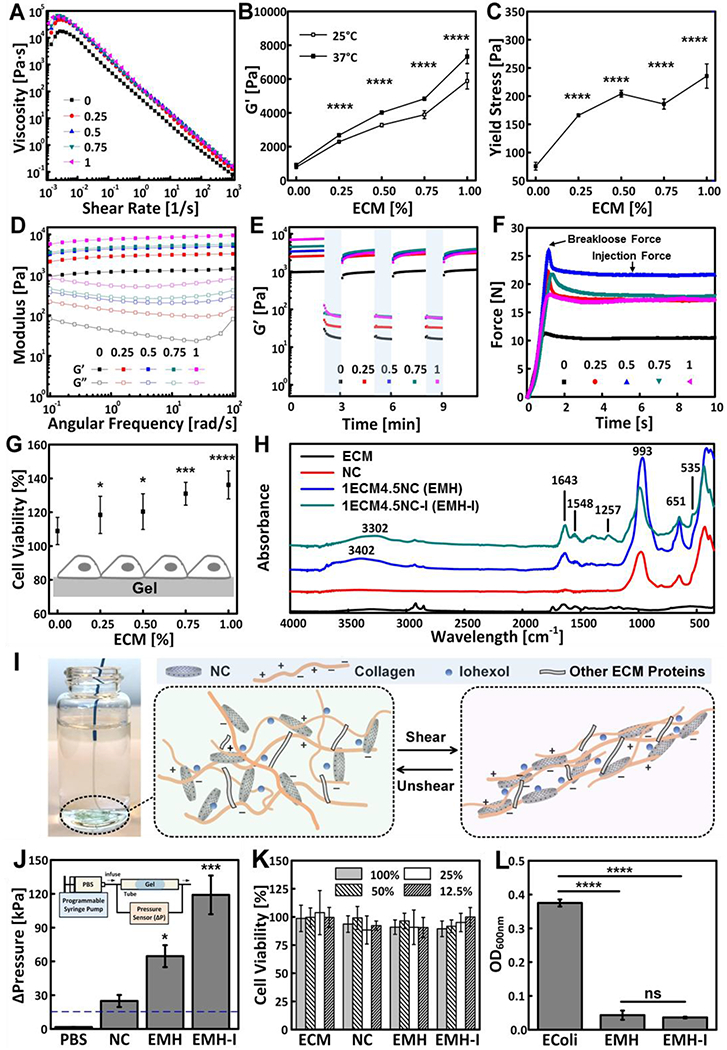
Mechanical properties and biofunctionalities of ECM-NC nanocomposite hydrogels. Rheology of xECM4.5NC, as characterized by (A) Shear rate sweeps. (B) G’ measured from oscillatory strain sweeps performed at 10 rad s−1 (n=3). (C) Yield stress calculated from oscillatory strain sweeps (n=3). (D) Oscillatory frequency sweeps performed at 0.1 % strain; (E) Time sweep revealing recoverability of gels under alternating cycles between 2-minute low 0.1 % strain and 1-minute high 100 % strain at 10 rad s−1. (F) Representative injection force curves, showing breakloose and injection forces. (G) Cell viability of L-929 fibroblasts seeded directly on ECM gels 16 hours, showing enhanced cell viability with an increasing amount of ECM in the gels (n=8). (H) FTIR spectra of ECM, NC, EMH, and EMH-I, showing chemical composition. (I) EMH-I extruded from a 2.8 F catheter by manual injection and schematics showing interactions between ECM proteins, NC and iohexol network under shear. (J) The pressure required to displace control (PBS alone), NC, EMH, and EMH-I (n=3). Inset shows the schematics of tube-based setup for in vitro occlusion assessment. (K) Viability of L-929 cells after incubated with ECM, NC, EMH, and EMH-I extractions at different concentrations for 24 hours, showing no toxicity of the gels (n=12). (L) Antibacterial properties tested by measuring the optical density of E.coli suspensions that were cultured on EMH and EMH-I (n=8). Unless otherwise stated, all experiments on gels were performed at 37 °C. ns, not significant; *p < 0.05, **p < 0.01, ****p < 0.0001. Each data point represents average ± standard deviation.
