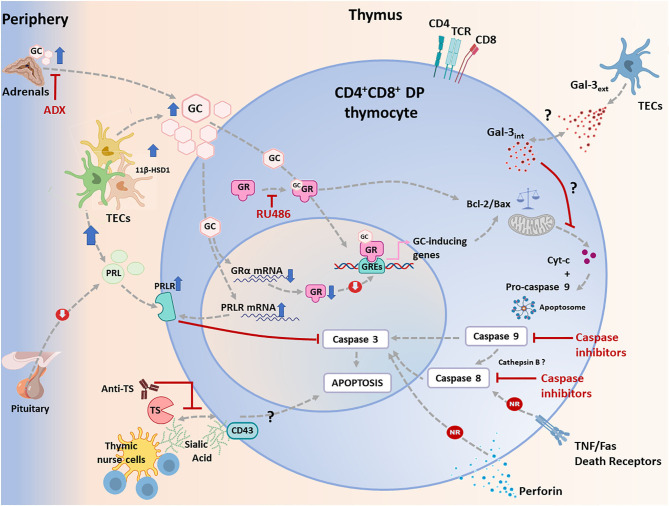
Figure 1.
Apoptotic signaling pathways triggered in CD4+CD8+ double positive (DP) thymocytes during T. cruzi infection in mice. Apoptosis can be initiated via two different routes including extrinsic and intrinsic pathways, which converge in a caspase activation cascade. The most relevant physiological way involved in T. cruzi-induced apoptosis of developing CD4+CD8+ DP thymocytes is linked to the systemic rise of glucocorticoid (GC) levels secondary to the host's stress response to the infection. This process is synergized by an increase in intrathymic production of GC by thymic epithelial cells (TECs), conjointly with an increased availability of induced-11 b-hydroxysteroid dehydrogenase(11β-HSD)-1. As proved, GC-driven apoptosis is greatly prevented by adrenalectomy or by the blockade of GC receptor (GR) with RU486. In addition, GC can stimulate the intrinsic pathway either genomically or non-genomically, a process that involves proteins of the Bcl-2 family. Pro-apoptotic and anti-apoptotic proteins of the Bcl-2 family are up-and down-regulated respectively, leading to the release of cytochrome c, which then forms the apoptosome together with pro-caspase 9 and Apaf-1, leading to the activation of caspase 9. This enzyme then activates caspase 3. During T. cruzi infection, cytochrome c is enhanced, and caspase 9 is activated in CD4+CD8+ DP thymocytes. As shown, inhibition of caspase 9 weakens CD4+CD8+ thymocyte death. In parallel, increased systemic and intrathymic GC production leads to a diminution of GR expression, probably as a negative regulatory loop tending to control unnecessary harmful effects upon the gland. Moreover, T. cruzi infection induces the synthesis of prolactin (PRL) by TECs, while GC improved the expression of the PRL receptor (PRLR) in TECs. Increased PRLR signaling then counterbalances GC-induced effects, preventing caspase 3 activation. Extrinsic signals can be initiated by cell death ligands (FasL and TNF-α) and involve caspase 8, but these routes seem to be non-relevant (NR) during T. cruzi infection, since atrophy takes also place in TNFR1+2 double knock-out mice or Fas knock-out animals. Similar results were observed in perforin knock-out animals. Since caspase 8 is also active, a possible alternative route for its activation may involve cathepsin B, but this route deserves confirmation. Yet, it is clear that caspase 8 inhibition reduces thymocyte death during T. cruzi infection. Thymocyte apoptosis can also be triggered by trans-sialidase (TS), a T. cruzi derived enzyme, and this effect is partially blocked by anti-TS antibodies against. Trans-sialidase transfers sialic acid residues between the parasite and thymocytes, and probably acts through CD43. The subsequent steps leading to apoptosis are unknown. Other pathways described, as caused by intracellular and extracellular Gal-3, seems to influence the intrinsic apoptotic pathway.