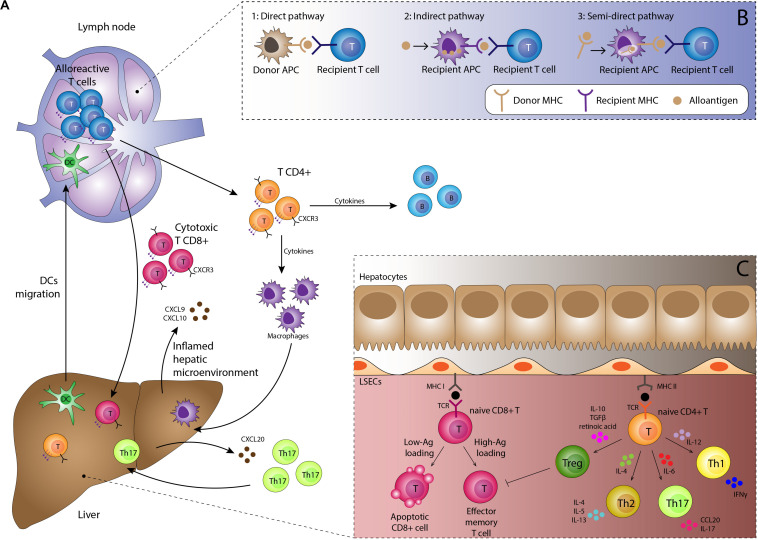
FIGURE 2.
(A) Immunological basis of TCMR. Activated dendritic cells migrate to lymphoid tissue presenting alloantigen on MHC class I and II molecules. Interaction with naïve alloreactive T-cells in the presence of appropriate co-stimulatory molecules and a pro-inflammatory cytokine milieu results in proliferation of alloreactive CD4 + and CD8 + effector T-cells and subsequent B-cell proliferation. Migration to the liver is orchestrated by chemokines such as CXCL9 and CXCL10 interacting with the CXCR3 receptor on lymphocytes in addition to complex interactions with the unique immunomodulatory liver sinusoidal endothelial cells. Lymphocyte subsets such as Th17 cells have specific recruitment mechanisms, providing potential therapeutic targets. Cells of the innate immune system including macrophages, neutrophils and eosinophils are recruited to the liver and along with the effector T-cells mediate tissue damage, resulting in the clinical manifestations of TCMR. (B) Three pathways for antigen presentation. The strongest alloimmune response is generated by the direct pathway which occurs in the early post-transplant period. Upon activation, donor-derived dendritic cells migrate to the lymph node, displaying non-self donor antigens within non-self donor MHC molecules. This provides potent stimulation for the mounting of a rejection response. The indirect and semi-direct pathways involve recipient-derived dendritic cells displaying self and non-self MHC molecules respectively. Whilst not as potent as the direct pathway, they are still able to sustain ongoing rejection. (C) Antigen presentation within the liver generally promotes tolerogenic responses. Antigen is also presented within the allograft by endothelial cells, macrophages, hepatic stellate cells, hepatocytes and biliary epithelium, with increased presentation seen during episodes of inflammation. However, most interactions with naïve lymphocytes within the liver result in tolerance rather than rejection, with apoptosis of effector cells and a skewing of T-cell differentiation toward the regulatory T-cell phenotype through an immunosuppressive cytokine profile.