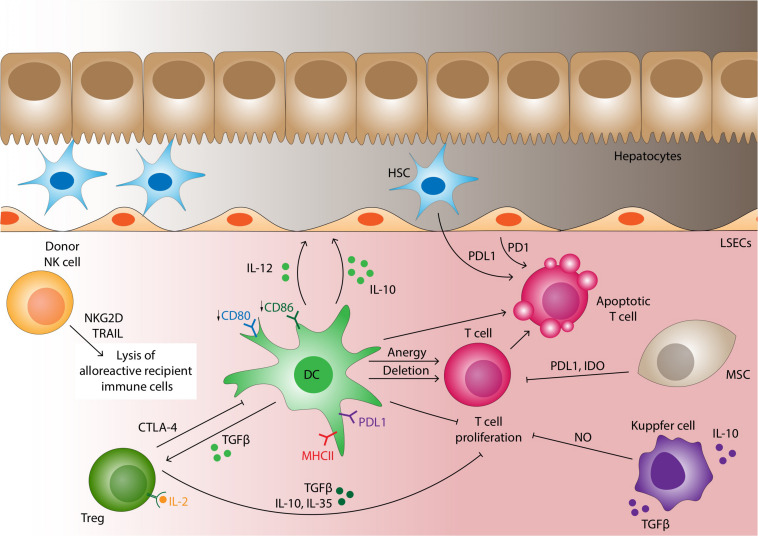
FIGURE 4.
Cellular mechanisms of liver allograft tolerance. A number of cells are able to promote tolerance in the liver allograft. Under normal non-inflamed conditions dendritic cells express low level of co-stimulatory molecules which along with the high expression of PDL1 induces T-cell anergy or deletion of the alloreactive T-cell clone. DCs also promote tolerance by secreting IL-10 and TGF-β which induces the differentiation of Tregs. CTLA4 on Tregs surface binds B7 on DC with a higher affinity than CD28, impairing DC-T-cell interactions. Tregs also contribute to the tolerogenic microenvironment also secreting TGF-β, IL-10 and IL-35, binding IL-2 on CD25 with higher affinity then T effector cells and by direct cytotoxicity through granzyme, perforin and Fas-FasL pathway. In contrast to recipient derived NK cells which tend to mediate rejection, donor derived NK cells transplanted as passenger cells within the liver allograft are able to directly lyse alloreactive recipient immune cells via NKG2D-MIC-A and TRAIL-TRAILR interactions leading to caspase-induced cell death. Mesenchymal stromal cells (MSCs) suppress T-cell proliferation and differentiation through the IDO pathway and cell-cell contact mediated by PDL1. Kupffer cells may be polarized to the M2 phenotype producing IL-10 and TGF-β and thus promoting tolerance. They can also release NO if stimulated by IFN-γ to inhibit T-cell proliferation. LSEC acts as non-professional antigen presenting cells with generally low levels of MHC class II expression; under many conditions induces antigen-specific tolerance. LSEC along with hepatic stellate cells induce T-cell apoptosis through PDL1/PD1 pathway interactions.