Fig. 4. MEKK2 counter-regulates CPR1-mediated SUMM2 ubiquitination and degradation.
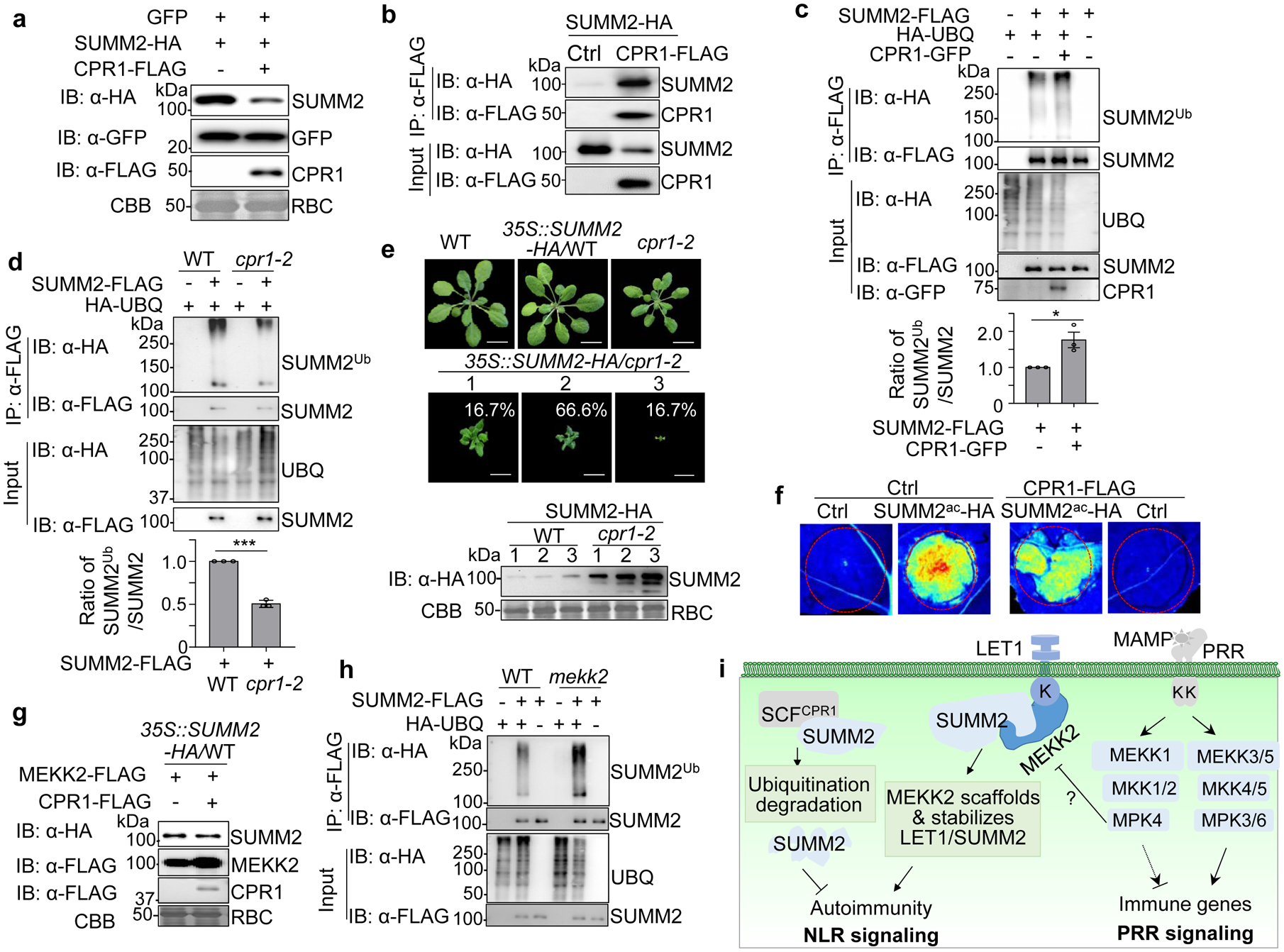
a, CPR1 promotes SUMM2 protein degradation. SUMM2-HA was co-expressed with a vector or CPR1-FLAG in N. benthamiana. GFP was included as a control. Total proteins were immunoblotted by an α-HA, α-FLAG or α-GFP antibody (top three panels). CBB is a loading control.
b, SUMM2 associates with CPR1. SUMM2-HA was co-expressed with a vector (Ctrl) or CPR1-FLAG in WT protoplasts. Total proteins were immunoprecipitated with α-FLAG affinity beads and then immunoblotted by an α-HA or α-FLAG antibody (top two panels). Immunoblots using total proteins before immunoprecipitation are shown as protein inputs (bottom two panels).
c, CPR1 enhances SUMM2 ubiquitination. SUMM2-FLAG was co-expressed with HA-UBQ with or without CPR1-GFP in WT protoplasts. Total proteins were immunoprecipitated with α-FLAG affinity beads and then immunoblotted by an α-HA or α-FLAG antibody (top two panels). Immunoblots using total protein before immunoprecipitation are shown as protein inputs (bottom three panels). The input for SUMM2 was adjusted to the similar level for Co-IP and IB. The quantification of SUMM2 ubiquitination with and without CPR1-GFP is shown on the bottom, P = 2.41 × 10−2. Data are shown as mean ± SE from three independent repeats. The asterisk indicates statistical significance by using two-sided two-tailed Student’s t-test (*, P < 0.05).
d, The cpr1–2 mutant reduces SUMM2 ubiquitination. Ubiquitination assay was performed as in c with protoplasts from WT and cpr1–2. The input for SUMM2 in WT and cpr1–2 was adjusted to a similar level for Co-IP and IB. The quantification of SUMM2 ubiquitination is shown on the bottom, P = 3.36 × 10−5. Data are shown as mean ± SE from three independent repeats. The asterisks indicate statistical significance by using two-sided two-tailed Student’s t-test (***, P < 0.001).
e, Overexpressing SUMM2 aggravates growth defects in cpr1–2. The 35S::SUMM2-HA construct was transformed into WT and cpr1–2. Plants were grown at 26 °C to reduce the growth defects of cpr1–2. All transgenic plants of 35S::SUMM2-HA/WT were phenotypically similar to WT (n>200). Total 120 transgenic plants of 35S::SUMM2-HA/cpr1–2 were grouped into three categories (the ratio of each category is indicated) based on the severity of growth defects. Pictures were taken four weeks after germination. Immunoblots with an α-HA antibody show SUMM2-HA protein expression, and CBB was used as a loading control (bottom). Scale bar, 1 cm.
f, CPR1 attenuates SUMM2ac-triggered cell death. SUMM2ac-HA was co-expressed with or without CPR1-FLAG in N. benthamiana. The GFP construct was used as a Ctrl. The images were taken two days after infiltration under the UV light.
g, MEKK2 antagonizes CPR1-mediated SUMM2 protein degradation. MEKK2-FLAG was co-expressed with or without CPR1-FLAG in protoplasts of 35S::SUMM2-HA transgenic plants.
h, Elevated SUMM2 ubiquitination in mekk2 compared to WT plants. SUMM2-FLAG was co-expressed with or without HA-UBQ in protoplasts of WT and mekk2. IP and IB were performed similarly as in c. The input for SUMM2 in WT and mekk2 was adjusted to a similar level for Co-IP and IB.
i, A model for MEKK2 scaffolding LET1-SUMM2 complex and protecting SUMM2 from CPR1-mediated degradation in cell death control. MAMP-activated MEKK1-MKK1/2-MPK4 cascade regulates PRR-mediated immune signaling and suppresses SUMM2-mediated autoimmunity via modulating MEKK2 protein. MEKK2 scaffolds and stabilizes LET1-SUMM2 complex, which blocks SUMM2 ubiquitination and degradation mediated by SCFCPR1 complex.
All the experiments were repeated at least three times with similar results.
