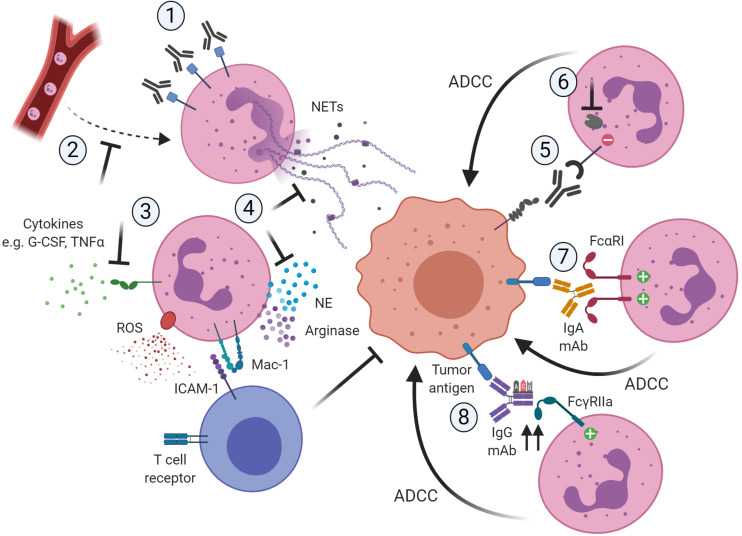
FIGURE 1.
Potential ways to therapeutically target neutrophils in cancer by blocking their pro-tumor activity (1–4) or promoting their anti-tumor capacities (5–8). (1) Reduction of neutrophil numbers in the TME, for example by using antibodies targeting CD33 present on immunosuppressive neutrophils. (2) Blocking the recruitment of pro-tumor neutrophils to the TME, for example by inhibiting the chemokine receptor CXCR2. (3) Blocking of activation signals such as G-CSF or TNFα necessary for neutrophils to acquire a pro-tumor or immunosuppressive phenotype. (4) Neutrophils require ROS production and degranulation to exert their immunosuppressive role, which is dependent on close contact with T cells via Mac-1. Also, neutrophils rely on mechanisms such as NET formation and the release of granule components such as NE to exert their pro-tumor activity. Targeting these downstream mechanisms would limit the pro-tumorigenic activity of neutrophils. (5) Interfering with innate immune inhibitory checkpoints would restore antibody-mediated anti-tumor capabilities of neutrophils. (6) Targeting the recruitment and function of downstream regulators of the inhibitory receptor would further enhance antibody-mediated anti-tumorigenic capacities of neutrophils toward tumor cells. (7) The use of IgA-based therapeutic mAbs that can bivalently bind FcαRI on the neutrophil would induce stronger anti-tumor cytotoxic responses. (8) The use of protein engineering techniques to modify the Fc region of IgG therapeutic antibodies would increase the affinity to the activating FcγRIIa, resulting in more potent ADCC responses toward the opsonized tumor cells. Created with BioRender.com.