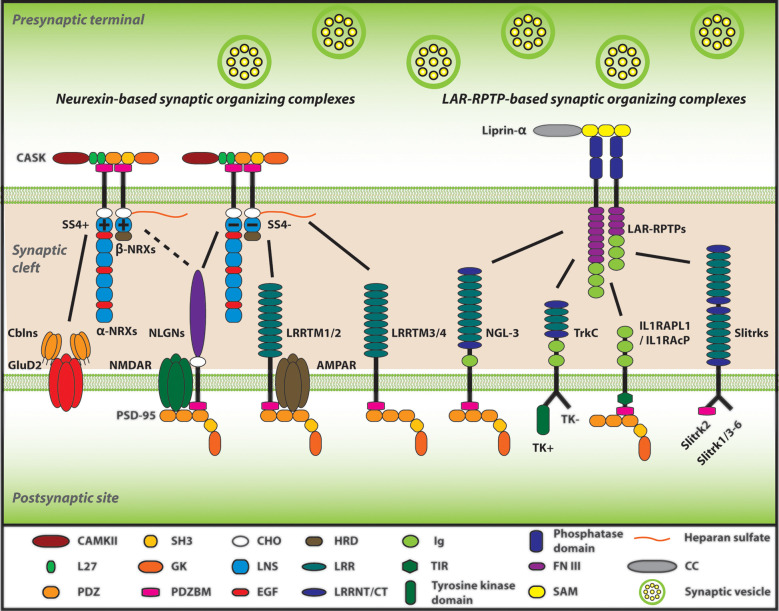
Figure 1.
Neurexins (NRXs) and Leukocyte common antigen-related receptor protein tyrosine phosphatases (LAR-RPTPs) serve as presynaptic hubs to orchestrate synapse organization. The trans-interaction between pre- and post-synaptic organizers generate retrograde and anterograde “synaptogenic” signals through the synaptic cleft, resulting in the presynaptic organization, including synaptic vesicles clustering, and in the postsynaptic organization, encompassing the recruitment of neurotransmitter receptors [e.g., AMPA-type and NMDA-type glutamate receptor (AMPAR and NMDAR)] and scaffold proteins (e.g., PSD-95). As presynaptic molecular hubs, NRXs and LAR-RPTPs trans-synaptically interact with multiple specific postsynaptic organizers, for instance, NRXs-neuroligins (NLGNs), NRXs-leucine-rich-repeat transmembrane neuronal proteins (LRRTMs), PTPσ-neurotrophin receptor tropomyosin-related kinase C (TrkC), PTPσ/δ-Slit and Trk-like proteins (Slitrks), along with others. Furthermore, SS4 insertion modulates NRX interactome by regulating its binding properties with its diverse ligands. Solid lines indicate protein interactions, and the dashed line indicates that the insertion of SS4 in NRXs weakens NRX-NLGN interaction.