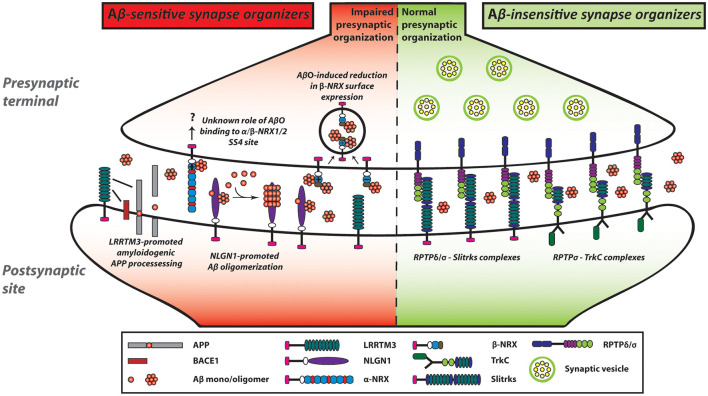
Figure 3.
NRX-based and LAR-RPTP-based synaptic organizing complexes display contrasting Aβ sensitivity. AβOs exert a pathological influence on NRX-based synaptic organizing complexes. In contrast, LAR-RPTPs and their post-synaptic binding partners appear to be resistant to AβO-induced deleterious effects on synapses. AβOs bind to the HRD of β-NRXs as well as to the SS4 of NRX1/2. Furthermore, AβOs reduce the expression of β-NRXs on axon surface in an HRD-dependent but SS4-independent manner. However, the role of the NRX SS4 in Aβ synaptic pathology remains to be understood. In addition to NRXs, NLGN1 can interact with Aβ and act as an Aβ deposition stabilizer to accelerate Aβ oligomer formation and fibrillar aggregation. Although AβOs affect not only NRXs but also LRRTMs and NLGNs, we propose that AβO-mediated β-NRX surface reduction may account the most in Aβ-impaired synapse organization. As an additional role of LRRTMs in the Aβ pathway, LRRTM3 interacts with amyloid precursor protein (APP) as well as β-site APP cleaving enzyme 1 (BACE1) and promotes amyloidogenic APP processing to enhance Aβ production. Thus, NRXs and their partners are highly linked with Aβ pathology, which may result in Aβ vulnerability of synapses in Alzheimer’s disease (AD). In contrast, AβOs do not interact with LAR-RPTPs or their postsynaptic partners including TrkC and Slitrks. Accordingly, LAR-RPTP-based complexes may contribute to making synapses more tolerant of Aβ pathology in AD. Solid lines indicate protein interactions.