Abstract
Current study was planned to explore the therapeutic response of different doses of hydroethanolic extract of Epimedium grandiflorum leaves in male albino rats. Phytochemical analysis, HPLC and FTIR spectroscopy results revealed the presence of wide range of phenolic compounds and functional groups, respectively. Further, extract not induced significant hemolysis (7.56 ± 1.297%) against PBS (3.65 ± 0.35%) as negative control; while have significant clot lysis (44 ± 5.2%) potential, exhibited DPPH (78.87 ± 5.427%) scavenging, H2O2 (31.82 ± 3.491%) scavenging, antioxidant and reducing power activities. In vivo experimentation in albino male rats’ revealed that administration of different doses (50, 100, 200 mg/Kg b.w.) of extract orally for 42 days after CCl4 intoxication significantly (P < 0.05) restore the selected parameters including liver enzymes, renal profiles, and stress markers and significantly (P < 0.05) increased reproductive hormones like testosterone, luteinizing hormone, follicle stimulating hormone and prolactin while significantly (P < 0.05) decreased progesterone and estradiol toward normal in dose dependent manner. Significant (P < 0.05) improvement in the structural architecture of testicular tissue particularly in high dose group (200 mg/Kg b.w.) was also observed. Results revealed E. grandiflorum has significant therapeutic response to address the healthcare problems particularly of impotency.
Keywords: phytoconstituents, doses, therapeutic response, antioxidant potential, reproduction, diseases
Introduction
Epimedium belongs to Berberidaceae family containing about 52 species, with multiple common names like Horny Goat Weed, Rowdy Lamb Herb, Xianlinpi, Bishop’s Hat, Fairy Wings, Barrenwort, and Yangheye or Yin Yang Huo.1 E. grandiflorum and Epimedium sagittatum are the most important Epimedium species used to enhance sexual performance. Due to aphrodisiac properties induction in Chinese goat, E. grandiflorum is commonly known as horny goat weed. Icariin, a flavonol glycoside as active ingredient of this plant induce the nitric oxide (NO) synthesis in penis,2 cavernosal smooth muscle where NO acts as inhibitor of cGMP-specific phosphodiesterase type 5 (PDE5),3 and boost the proliferation of smooth muscle, and reduce the formation of advanced glycation end product4 and caricaturist endogenous androgens.5 These beneficial effects of Epimedium species are due to the presence of various phytochemical constituents; and about over 260 including alkaloids, flavonoids, proteins, glycosides, bioactive peptides, waxes, amino acids, phyto-hormones are responsible for beneficiary activities, have been isolated. The active compounds of Epimedium possess a wide range of pharmacological actions including the regulation of hormones, strengthening yang, to cure osteoporosis, boosting immunological function, hepatoprotective, anti-cancer, anti-oxidation, anti-lipidemic, anti-aging, and anti-depressant activities. Further, various in vivo and in vitro studies have been done to screen the pharmacological activities due to effective monomeric compounds or active parts present in Epimedium.1
Infertility can also be referred as biological failure of an individual to contribute to conception which might be due to male or female. It is becoming one of the major problems of our society and about 13-18% married couples’ i.e 1 in 6 couples are affected by infertility.6 It was reported by WHO that infertility in worldwide affects about 60-80 million couples at reproductive age.7 Both in male and female dysregulation of reproductive hormones or anatomical anomalies might be the major causes of infertility.8 Hypothalamus-pituitary-gonadal axis and various factors like behavioral, environmental, genotoxic and genetic factors compromise the spermatogenesis in male.9 Phytotherapeutic approaches to treat infertility motivated to explore numerous plants and polyherbal preparations because modern drugs development is expensive and time consuming with many side effects. So in developing countries, traditional medicine plays major role in healthcare community10 and different selected parts like fruits, seeds, leaves, roots, etc or their extract powders are used to treat various diseases of humans, animals and plants.11 In addition medicinal plants used as traditional medicine are easily consumable, easily available and cheaper with simple preparation strategies to mediate beneficial effects.12,13 Moreover, it was reported that 80% population worldwide and more than 30% of pharmaceutical formulations are dependent upon medicinal plants, reported by WHO.14 The current study was planned with the main objectives to determine the phytochemical constituents of E. grandiflorum leaves aqueous hydroethanolic extract, therapeutic response by determining the antioxidant parameters and reproductive hormones as well as structure of testicular tissue in CCl4 intoxicated male albino rats.
Materials and Methods
Selection and Collection of Plant Material
E. grandiflorum leaves were purchased from local market of Faisalabad-Pakistan and were taxonomically identified and confirmed from Department of Botany, Government College University, Faisalabad-Pakistan. It was well reported that ethanol and methanol act as effective solvents for the extraction of antioxidant phenolic compounds.15 So aqueous Ethanolic (30:70) plant extract was prepared and dry leaves were sonicated twice with 10 time’s higher volume of hydro alcohol for 48 hours at room temperature. Then the solution was filtered by Whatman filter paper number 1 and evaporated using rotary evaporator under reduced pressure.
Phytochemical Analysis
Qualitative analysis
Various phytochemicals including alkaloids, flavonoids, tannins, saponins, glycosides, steroids and triterpenoids were investigated in the hydroethanolic E. grandiflorum extract by standard methods.16-19
Quantitative Estimation of Phytochemicals
Total phenolic contents (TPC) and Total flavonoids contents (TFC)
Folin-Ciocalteu method was used to quantify the TPC present in hydroethanolic extract of E. grandiflorum according the procedure described by Jain et al., and gallic acid as standard was used.16 The contents of total phenolics in plant extract were quantified as milligram (mg) gallic acid equilant (GAE) per milliliter (mL) of plant hydroethanolic extract. While TFC was measured using the method explained by Pranuthi et al.17 Catechin as standard was used and TFC represented as µg CE/g (microgram Catechin per gram) of dried plants material.
High performance liquid chromatography (HPLC) analysis
HPLC was used to determine the phenolic compounds following the method with some modifications described by Xie et al.20 C18 column (250 × 4.6 mm internal diameter), having 5 µm film thickness, accompanying an oven set at 30°C was used in Liquid chromatography. Chromera HPLC system (Perkin Elmer, USA.) attached with Flexer Binary LC pump, UV/Vis LC Detector (Shelton CT, 06484 USA) controlled by software V. 4.2. 6410 used to analyze the data. Two solutions including solvent A consisted of acetonitrile: methanol (70:30) and solvent B composed of double distilled water with 0.5% glacial acetic acid were used as the mobile phase. UV spectra were recorded at 275 nm. Different phytocompounds were identified using 275 nm wavelength by comparing the retention times and spiking samples with standards.
Fourier-transform infrared (FTIR) spectroscopy
E. grandiflorum extract was milled with potassium bromide (KBr) powder and pellets were prepared by pressing. After making the pellets FTIR spectrometer (Model Bruker Platinum ATR with accessories A225/Q Platinum ATR Multiple Crystals CRY diamond and having Interferogram Size of 10550 points) in the frequency range of 400-4,000/cm was used to analyze for the identification of functional groups as representative of wide range of important phytoconstituents.
Antioxidant Profiling
Total antioxidant capacity (TAC) by phosphomolybdenum method
Phosphomolybdenum method to determine the total antioxidant capacity (TAC) of the hydroethanolic extract of E. grandiflorum was used, following the procedure described by Prieto et al.21 Different concentrations of ascorbic acid were mixed in absolute methanol to make standard solutions (1000, 500, 250, 125, 62.5 and 31.25 μg/mL). The total antioxidant activity is expressed as the number of gram equivalent of ascorbic acid.
DPPH radical scavenging potential
To evaluate the antioxidants potential of plant extract the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay was used as described by Shahid et al.22 The percent DPPH inhibition was calculated using the following formula
Where AS = Absorbance of Sample, A0 = Absorbance of Blank.
Measurement of reducing power by FRAP method
Fe3+ (CN)6 reduction into Fe2+ (CN)6 was used to measure the reducing potential of hydroethanolic extract according to Yadav et al.23 The absorbance at 700 nm wavelength represented the reductive potential of sample.
Hydrogen peroxide (H2O2) scavenging capacity
The H2O2 scavenging capacity of E. grandiflorum hydroethanolic extract was measured following the protocol described by Ruch et al.24 Ascorbic acid used as standard and % age H2O2 scavenging activities were calculated as
Where AC = absorbance of the control, AS= absorbance in the presence of the sample of extract or standards.
Hemolytic Assay to Determine Cytotoxic Potential of Plants Extract
In vitro the cytotoxic potential of hydroethanolic plant extract, 10 mg/mL was dissolved in 20% DMSO (Dimethyl sulfoxide), was determined through hemolytic assay performed by spectrophotometric method against Human erythrocytes.Triton-x-100 as positive control and phosphate buffer saline (PBS) as negative control were used.25- 26 % age hemolysis was calculated as:
Here: At = Test sample absorbance, An = Saline control absorbance, Ac = The DMSO control absorbance.
Clot Lysis Assay to Determine Thrombolytic Activity of Plant Extract
Clot lysis method was used to evaluate the in vitro thrombolytic potential of hydroethanolic extract following the procedure of Rahman et al.27 Streptokinase and PBS were used as positive and negative controls, respectively. The % age clot lysis activity was calculated as:
Clot lysis (%) = (clot weight before lysis − clot weight after lysis) / clot weight before lysis × 100
In Vivo Experimentation
Animal grouping and different dosage plan
The male albino rats weighing about 160 gram to 190 gram of 8-10 weeks of age were used as animal model for in vivo experimentation and all the animals (n = 35) were divided into different groups with 5 animals (n = 5) in each group as follow Group 1 (GM-1): Healthy Control (Normal diet and water administered only); Group 2 (GMT-2): Intoxicated control (20% CCl4 0.5 mL/Kg body weight (b.w) intraperitoneally (i.p.) twice a week); Group 3A (GMT-3A): Positive control (20% CCl4 0.5 mL/ Kg b.w i.p. twice a week and Injected Testosterone enanthate 2.5 mg/Kg b.w intra muscular (i.m) twice a week); Group 3B (GMT-3B): Positive control (20% CCl4 0.5 mL/ Kg b.w i.p. twice a week and given Laboob-e-Kabeer (Polyherbal preparation) 200mg/kg orally twice a week); Group 4A (GMT-4A): 20% CCl4 0.5 mL/ Kg b.w i.p. twice a week and hydroethanolic plant extract (50mg/Kg b.w. as low dose) orally daily; Group 4B (GMT-4B): 20% CCl4 0.5 mL/ Kg b.w i.p. twice a week and hydroethanolic plant extract (100mg/Kg b.w. as intermediate dose) orally daily; and Group 4C (GMT-4C): 20% CCl4 0.5 mL/ Kg b.w i.p. twice a week and hydroethanolic plant extract (200mg/Kg b.w. as high dose) orally daily. All experimental animal groups were kept at normal diet and 12 hours light/dark cycle with normal husbandry environment for 6 weeks in the animal house, Department of Physiology, Government College University, Faisalabad-Pakistan after institutional ethical review committee.
Cytotoxic Studies
To evaluate the toxicity of hydroetaholic extract in rats’ cytotoxic studies were carried out by increasing the dose from 500 mg/kg b.w. to 750 mg/kg b.w. to 1000 mg/kg b.w. It was reported that at the dose of 1000 mg/kg toxic clinical features appeared in 12 hours of drug administration. Therefore, highest dose was selected as less than one fourth (200mg/kg b.w.) of toxic level drug to run the trial.
Change in body weight and collection of blood samples
At the final day of experiment to determine the therapeutic response of plant extract, rats of each group were weighed to evaluate the change in body weight. Gain in body weight (grams) was evaluated by subtracting the pre-clinical trial body weight from post-clinical trial body weight of each group. For the determination of selected antioxidant and biochemical parameters heart puncture technique after 42 days was performed to collect blood samples. Serum from non-anti-coagulated blood was separated after centrifugation and frozen at -20 °C until analysis.
Stress markers and biochemical parameters estimation
Serum stress markers including total antioxidant status (TAS), total oxidant status (TOS), Malondialdehyde (MDA) by Sahreen et al28; Superoxide dismutase (SOD) by Kakkar et al,29 and Catalase (CAT) by Chance and Maehly30 were determined. To evaluate the safety concerns of hydroethanolic plant extract liver enzymes like transaminases (ALT and AST) by Bergmeyer et al.,31 lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) were determined colorimetrically as described by Thomas,32 total protein, albumin, globulin by Koller et al.,33 and renal profile including urea by Burtis and Ashwood,34 creatinine and uric acid by Swanson et al.,35 were determined using automated photometric methods. The reproductive hormones including testosterone by Chen et al., (1991),36 Luteinizing hormone (LH) by Frank et al., (1996),37 Follicle Stimulating hormone (FSH) by Qiu et al., (1998),38 Prolactin by Vanderpump et al., (1998),39 progesterone by Radwanska et al., (1978)40 and estradiol by Tsang et al., (1980)41 were measured using standard Enzyme Linked Immunosorbent Assay (ELISA) based kit method.
Histological examination of testicular tissue
Tissue samples were collected in buffered formalin (neutral) container after dissecting the animals for histological examination. After the fixation sections were taken in labeled tissue cassettes and processed to embed in paraffin wax adopting the protocol described by Bancroft and Gamble.42 After embedding the tissue in paraffin wax, microtomy was performed to take thin sections and after deparaffinization, sections were stained using Hematoxylin and Eosin (H & E) stain according to the standard procedure of Bancroft and Gamble,42 and observed under microscope.
Statistical Analysis
The obtained results were represented as Mean ± SEM and further interpreted through applying 1 way ANOVA test and to determine the difference between groups pairwise study was determined by Tukey’s test using Minitab 17 statistical software (trial version).43
Results
Phytochemical Constituents and Antioxidant Potential
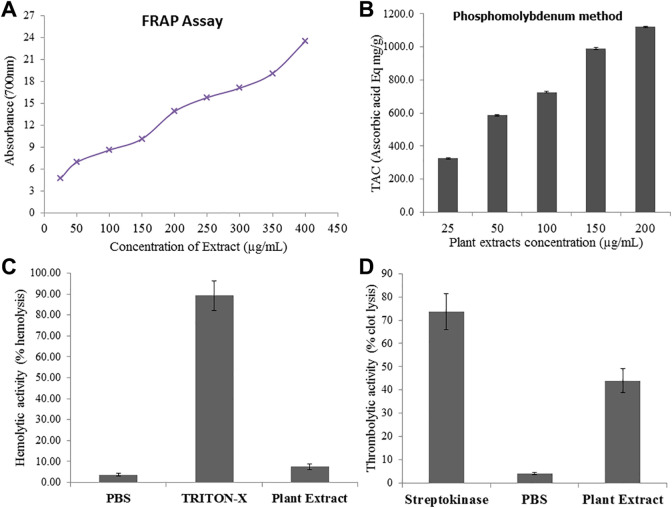
Results of qualitative analysis explored different phytochemical constituents like alkaloids, flavonoids, tannins, saponins, glycosides, steroids and triterpenoids in the hydroethanolic extract of E. grandiflorum leaves extract (Table 1). The results of quantitative analysis revealed the total flavonoid (241.8 ± 11.18 µg CE/g dry plants material) and phenolic contents (1010.72 ± 12.49 mg GAE/g dry plants material) in current study and the mean ± SE (Standard error of mean) are given in Table 2. Free radical scavenging potentials determinations are very important parameters to evaluate the antioxidant capacity of medicinal plants. Ferric Reducing Antioxidant Power (FRAP) Assay, Phosphomolybdenum, DPPH (1,1-diphenyl-2-picrylhydrazyl) and hydrogen peroxide scavenging assays were used to evaluate the antioxidant potential of hydroethanolic plant extract and the results represented in Table 2 and Figure 1A and B. The results revealed significant (p < 0.05) increase in radical scavenging and reducing activity directly correlated with the plant extracts concentrations (Figure 1A and B).
Table 1.
Qualitative Phytochemical Screening of Ethanolic Extract of E. grandiflorum.
| Plants/phytochemicals | Epimedium grandiflorum |
|---|---|
| Alkaloids | + |
| Flavonoids | ++ |
| Tannins | + |
| Saponins | + |
| Glycosides | ++ |
| Steroids | ++ |
| Triterpenoids | + |
(+) indicates the presence of phytoconstituents, (-) indicates no phytoconstituents present
Table 2.
Total Phenolic, Total Flavonoid Contents, and Antioxidant Activities of Ethanolic Extract of E. grandiflorum (Mean ± SEM).
| Plants/phytochemicals | Epimedium grandiflorum |
|---|---|
| TPC (mg GAE/g dry plants material) | 1010.72 ± 12.49 |
| TFC (µg CE/g dry plants material) | 241.8 ± 11.18 |
| DPPH Scavenging activity (%) | 78.87 ± 5.427 |
| H2O2 Scavenging activity (%) | 31.82 ± 3.491 |
Figure 1.
(A) Reducing potential of hydroethanolic extract of E. grandiflorum using different concentrations by FRAP method, (B) Total antioxidant capacity of hydroethanolic extract of E. grandiflorum by Phosphomolybdate assay (C) Thrombolytic activity of hydroethanolic extract of E. grandiflorum measured against streptokinase as positive control and Phosphate buffer saline (PBS) as negative control (D) Hemolytic activity of hydroethanolic extract of E. grandiflorum measured against Triton-X as positive control and PBS as negative control.
HPLC and FTIR Spectroscopy
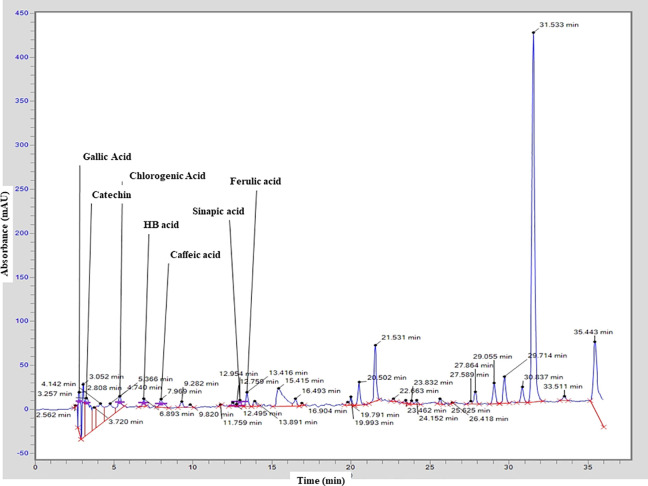
In the present study, HPLC-UV was used to analyze the hydroethanolic extract of E. grandiflorum leaves qualitatively. The results revealed the presence of different phenolic compounds might be responsible for strong antioxidant potential of E. genadiflorum includes Gallic Acid (Rt = 2.808), Catechin (Rt = 3.257), Chlorogenic acid (Rt = 5.366), HB acid (Rt = 6.893), Caffeic acid (Rt = 7.969), Sinapic acid (Rt = 12.954) and Ferulic acid (Rt = 13.416). The results of HPLC also revealed some peaks with different retention time could not be identified; however, based on their chromatographic behaviors and UV spectra, they may correspond to unknown flavonoids compounds as presented in respective chromatogram (Figure 2).
Figure 2.
Chromatogram of hydroethanolic extract of E. grandiflorum obtained from HPLC analysis representing the presence of different phenolic molecules.
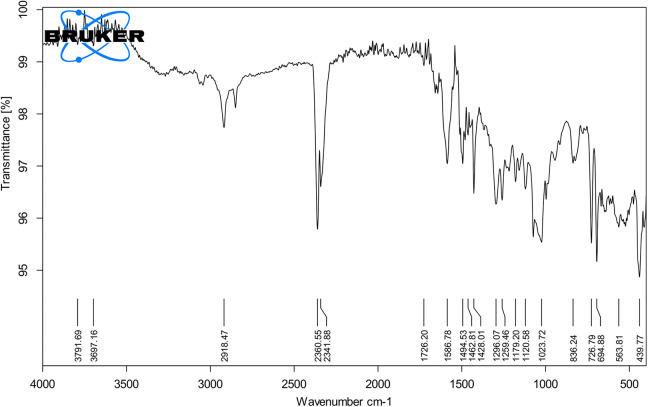
According to infrared spectroscopy in Figure 3, there were absorption peaks at 3,500-4,000/cm, 2,918/cm, 2,360/cm, 2,341/cm, 1,726/cm, 1,586/cm, 1,494/cm, 1,462/cm, 1,426/cm, 1,428/cm, 1,296/cm, 1,259/cm, 1,179/cm, 1,120/cm, 1,023/cm, 836/cm, 726/cm, 694/cm, 563/cm and 439/cm. The absorption peaks at range 3,500 to 4,000/cm was associated with the stretching vibration of O-H, which meant that there were hydrogen bonds in intramolecules and intermolecules. The absorption peak at 2,918/cm was attributed to the asymmetrically stretching vibration of C-H. The peak at 2,360/cm and 2,341/cm might be related to stretching vibration of C-H. The absorption peak at 1,726/cm was attributed to the symmetrically stretching vibration of C=O. The absorption peaks at 1,586/cm might be related to the stretching vibration of C=N and C=C. The peaks at 1400 to 1500/cm (1,494/cm, 1,462/cm, 1,426/cm and 1,428/cm) might be attributed with strong to medium vibration of C=C and N=O functional groups. The absorption peaks at 1,296/cm and 1,259 was due to the stretching vibration of C-O. The peaks at 1,179/cm and 1,120/cm were attributed to the asymmetrically stretching vibration of C-O-C. The absorbance peak at 563/cm might be represented of aliphatic C-I stretching while the peak at 439/cm might be related to S-S stretching vibration.
Figure 3.
Infrared spectra of the hydroethanolic extract of E. grandiflorum leaves revealing the presence of wide range of functional groups.
Hemolytic and Thrombolytic Activities
Results of hemolytic assay revealed that the %age (percentage) hemolysis of plant extract is significantly negligible as compare to positive control (Triton-X) shown in Figure 1C. To evaluate the blood and semen thinning effect of hydroethanolic extract clot lysis activity was measured using human clotted blood. Results revealed that Streptokinase (SK; 100 µL) incubation with clot for 90 min at 37ºC dissolve the 73.6 ± 7.7% clot while on the other hand only 3.65 ± 0.35% clot lysis was observed in clots treated with phosphate buffer saline (PBS) and significant (p < 0.001) thrombolytic activity of hydroethanolic extract of plant (44 ± 5.2%) was observed (Figure 1D).
In Vivo Hydroethanolic Extract’ Therapeutic Response
Hepatoprotective and renoprotective activities of E. grandiflorum extract
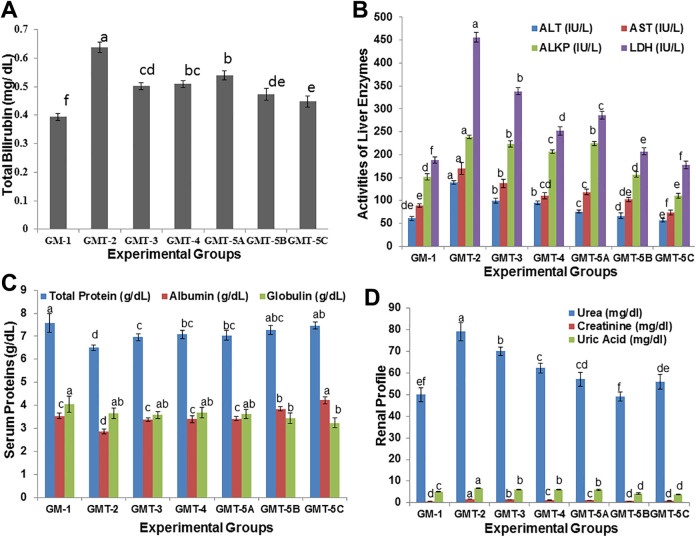
Evaluation of drugs toxicity is one of the major concerns in drug development44 because most of the herbal preparations have a wide range of health benefits so hepatoprotective and renoprotective aspects were also evaluated. The statistical analysis revealed that there was a significant (P < 0.01) increased in total bilirubin and liver enzymes on intoxication with CCl4 while serum proteins significantly (P < 0.05) decreased. On the other hands, Significant (P < 0.05) improvement was reported after administration of different doses of hydroethanolic extract of plant and this improvement in liver enzymes and proteins was in dose dependent manner. Although, the concentration of globulin also increases with increased in concentration of extract amount but a significant (P < 0.05) difference was reported on administration of 200 mg per Kg bw extract (Figures 4A-C). The intoxication with CCl4 administration significantly (P < 0.05) affects the kidney function evaluated by measuring blood urea, creatinine and uric acid level as compared to normal animal group while administration of hydroethanolic extract of E. grandiflorum significantly (P < 0.05) restore these renal parameters on dose dependent manner (Figure 4D).
Figure 4.
(A) Concentration of Serum Total Bilirubin (B) Activities of liver enzymes including alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALKP) and lactate dehydrogenase (LDH) (C) Concentration of serum proteins like Total proteins, albumin and globulin and (D) Concentration of blood urea, creatinine and uric acid in the blood samples collected from different experimental groups at the end of trial. Values are Mean ± SEM (standard error of Mean) of the study groups. Colum share the same alphabets indicate non-significant (P > 0.05) differences.
Total oxidant (TOS), antioxidant status (TAS) and stress markers in rats’ blood
It was noted that induction of CCl4 significantly (P < 0.01) decreased the TAS and increased the TOS in rats while the administration of plant extracts orally on daily basis revert significantly (P < 0.01) TAS and TOS (Table 3). The results revealed a significant (P < 0.05) high MDA level in CCl4 intoxicated rats as compare to other studied animals. MDA level returned to near normal after administration of E. grandiflorum extract in rats (Table 3). Moreover, SOD (Superoxide dismutase) and CAT (Catalase) as enzymatic antioxidant and decreased in both enzymes activities was also noted on intoxication with CCl4 while in plants extract and positive drugs treated rats, both SOD and CAT significantly (P < 0.05) improves in dose dependent manner (Table 3).
Table 3.
Effect of Selected Ethanolic E. grandiflorum Extract and Control Drugs on Stress Markers in Male Albino Rats.
| Parameters | GM-1 | GMT-2 | GMT-3 | GMT-4 | GMT-5A | GMT-5B | GMT-5C | P-value |
|---|---|---|---|---|---|---|---|---|
| TAS | 0.823 ± 0.051CD | 0.504 ± 0.038F | 0.718 ± 0.051E | 0.737 ± 0.044DE | 0.906 ± 0.026C | 1.473 ± 0.073B | 2.402 ± 0.037A | <0.01 |
| TOS | 15.758 ± 2.055BC | 27.748 ± 2.317A | 24.672 ± 2.320A | 23.940 ± 2.336A | 19.544 ± 1.547B | 15.905 ± 1.132BC | 12.089 ± 1.278C | <0.01 |
| MDA | 11.51 ± 1.55C | 18.28 ± 1.54A | 15.75 ± 1.27AB | 15.08 ± 1.17B | 12.33 ± 1.10C | 9.77 ± 0.85CD | 8.13 ± 1.31D | <0.01 |
| SOD | 37.85 ± 2.74CD | 26.92 ± 1.95E | 32.86 ± 2.58D | 36.20 ± 2.69CD | 38.53 ± 2.00C | 47.65 ± 2.37B | 55.68 ± 3.06A | <0.01 |
| CAT | 14.74 ± 2.07CD | 9.62 ± 1.59E | 11.32 ± 1.60DE | 15.54 ± 1.56C | 15.22 ± 1.60CD | 22.92 ± 2.23B | 27.85 ± 2.94A | <0.01 |
GM-1; normal control male rats given only diet, GMT-2; toxic group male rats intoxicated with 0.5 mL 20% CCl4, GMT-3; Positive control group male rats given 0.5 mL 20% CCl4 + 2.5 mg/Kg bw testosterone injection, GMT-4; Positive control group male rats given 0.5 mL 20% CCl4 + 200 mg/Kg bw Laboob e Kabir (Polyherbal Preparation) orally, GMT-5A, GMT-5B, GMT-5B; Test group male rats intoxicated with 0.5 mL 20 % CCl4 and administered with ethanolic extract E. grandiflorum in dose concentrations of 50, 100 & 200 mg per Kg bw, orally daily respectively TAS (Total antioxidant status), TOS (Total oxidant status), MDA (Malondialdehyde), SOD (Superoxide dismutase), CAT (Catalase) Values are mean ± SE (standard error) of means of the study groups. Different letters in superscripts in same row indicate significant group mean differences. The p < 0.05 considered statistically significant while p < 0.01 indicates highly significant.
Change in body, testis weight and reproductive hormones
The changes in the results, as mean ± SEM, of body weight (g), testis weight (g), relative gain in testis (g) and reproductive hormones in male rats have been given in Table 4 and Figure 5. The results of statistical test revealed that CCl4 exposure significantly (P < 0.01) decreased the body weight and reproductive hormones including testosterone, LH, FSH and prolactin while progesterone and estradiol increased significantly (P < 0.05) in rats in comparison with the normal rats (Table 4 and Figure 5). On the administration of different doses of hydroethanolic extracts significant (P < 0.01) restoration of these hormones in comparison of positive control animals administered testosterone injecction and Laboob-e-Kabir have been reported (Table 4 and Figure 5).
Table 4.
Effect of Ethanolic Plant Extract of E. grandiflorum and Control Drugs on Body Weight (g) and Testis Weight in Male Albino Rats.
| Parameters | GM-1 | GMT-2 | GMT-3 | GMT-4 | GMT-5A | GMT-5B | GMT-5C | p-value |
|---|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 160.6 ± 6.95B | 170 ± 6.44B | 170.8 ± 8.25B | 165 ± 5.79B | 187 ± 6.52A | 174.8 ± 9.68AB | 166.2 ± 8.87B | <0.01 |
| Final body weight (g) | 248 ± 9.083BC | 237.4 ± 9.274C | 264 ± 7.176AB | 250 ± 8.916BC | 277.8 ± 6.325A | 270 ± 8.396A | 271 ± 8.276A | <0.01 |
| Body weight gain (g) | 87.4 ± 3.962BC | 65.4 ± 4.159D | 93.8 ± 4.324B | 85 ± 3.050C | 90.8 ± 4.324B | 95.2 ± 4.637A | 104.8 ± 3.317A | <0.05 |
| Testis Weight Gain (g) | 4.472 ± 0.145C | 3.442 ± 0.178E | 4.588 ± 0.131C | 4.104 ± 0.073D | 5.058 ± 0.106B | 5.762 ± 0.183A | 6.034 ± 0.126A | <0.05 |
| Relative weight gain in Testis (g) | 1.804 ± 0.044BC | 1.455 ± 0.108D | 1.740 ± 0.095BC | 1.644 ± 0.073C | 1.827 ± 0.047B | 2.136 ± 0.100A | 2.229 ± 0.101A | <0.01 |
GM-1; normal control male rats given only diet, GMT-2; toxic group male rats intoxicated with 0.5 mL 20% CCl4, GMT-3; Positive control group male rats given 0.5 mL 20% CCl4 + 2.5 mg/Kg bw testosterone injection, GMT-4; Positive control group male rats given 0.5 mL 20% CCl4 + 200 mg/Kg bw Laboob e Kabir (Polyherbal Preparation) orally, GMT-5A, GMT-5B, GMT-5B; Test group male rats intoxicated with 0.5 mL 20 % CCl4 and administered with ethanolic extract E. grandiflorum in dose concentrations of 50, 100 & 200 mg per Kg bw, orally daily respectively Values are Mean ± SE (standard error) of means of the study groups. Different alphabetic letters in same row specify the significant differences in group means. The level of significance was considered at p < 0.05 while p < 0.01 indicates highly significant.
Figure 5.
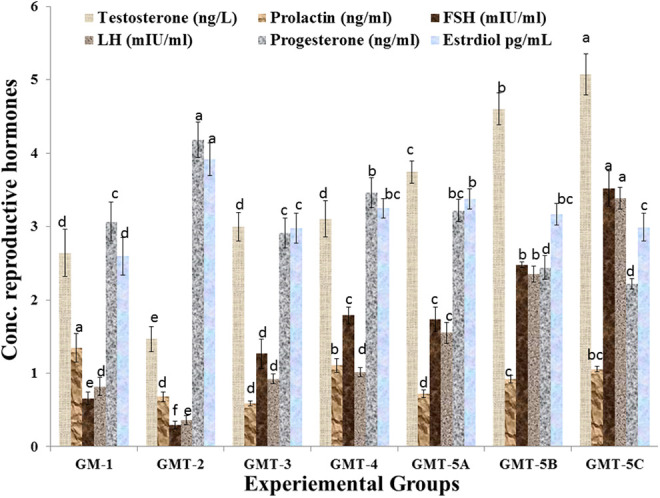
Concentration of reproductive hormones including testosterone, prolactin, FSH, LH, progesterone and estradiol in the blood samples collected from different experimental groups at the end of trial. Values are mean ± SE (standard error) of means of the study groups. Colum share the same alphabets indicate non-significant (p > 0.05) differences.
Histological examination of testicular tissue
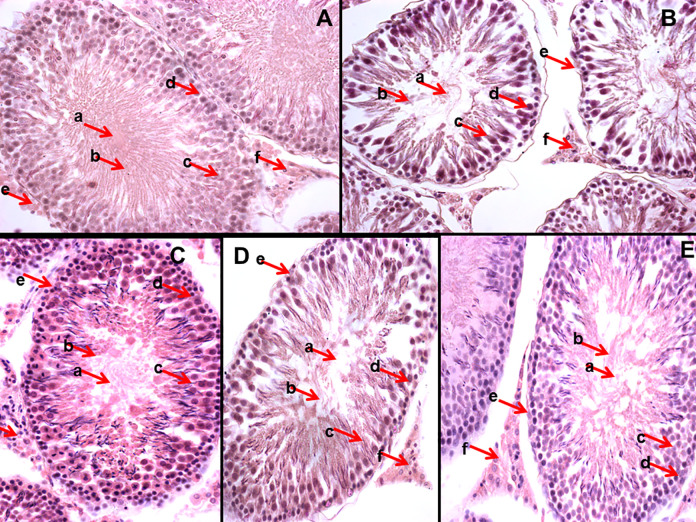
The histological examination of testicular tissues revealed that CCl4 intoxication of rats significantly damaged the spermatogenic cells in the seminiferous tubules (Figure 6B) as compared to normal control group, as normal testis are composed of multiple seminiferous tubular cells with germinal epithelium (4-6 layers) at various spermatogenic stages with mature spermatozoa in the central lumen. The other cells including sertoli cells and leydig cells are also present in adequate number in normal testis (Figure 6A). The sections from both positive controls and rats administrated hydroethanolic plant extract showed noticeable improvement in the histo-architecture after CCl4 intoxication (Figure 6C-E).
Figure 6.
Histological Sections taken from Testicular tissues of different experimental male rats and stained with H & E stain (A) GM-1 (B) GMT-2 (C) GMT-3 (C) GMT-4 (D) GMT-5B. The alphabets (a-f) indicates different histological features of testis as (a) represent the lumen of seminiferous tubule (b) shows mature spermatozoa (c) indicates primary spermatocyte (d) highlight the spermatogonium (e) marks the seminiferous tubules basement membrane and (f) represent the leydig cells present between seminiferous tubules spaces.
Discussion
Phytochemical Constituents and Antioxidant Potential
A wide range of medicinal plants are being used with several therapeutic potentials due to the presence of phytochemical constituents including alkaloids, flavonoids, steroids, glycosides, tannins and saponins.45 Natural alkaloid as well as their synthetic derivatives have proven to have wide range of pharmacological activities including antimicrobial, analgesic and antispasmodic46 and it was reported that the antioxidant potential of herbal preparations is due to the presence of phenolic compounds.47 Moreover, it was well reported that ethanol and methanol act as effective solvents for the extraction of antioxidant phenolic compounds15 and most important groups of natural products include flavonoids are the widespread class of natural phenolic constituents. It was reported that quenching of active oxygen species is due to several flavonoids present in extract.48
Defect in the natural antioxidant defense system might create oxidative stress which might be associated with different types of disorders and other healthcare problems49 and to prevent these oxidant associated disorders natural antioxidants including flavonoids, carotenoids as well as vitamins like A, C and E with food supplements are used.50
It was reported that E. grandiflorum has good antioxidant potential, and could act as natural antioxidants in pharmaceutical preparations, foods and cosmetics, and our results are in agreement with the findings of Yang et al.,51 who also found the good radical scavenging activity of Epimedium species. Moreover, results also explored that the increase in the reductive capacity is directly proportional to the concentration of plant extracts. In addition, it was also reported that the hydroethanolic extract of E. grandiflorum contains phyto compounds responsible for reducing potential, a significant indicator of its antioxidant capacity.52,53
Hemolytic and Thrombolytic Activities
Evaluation of drugs toxicity is one of the major concerns in drug development44 because most of the herbal preparations have a wide range of health benefits. Very high viscosity of semen is also a cause of infertility because this significantly affects the motility of spermatozoa. Although, a wide range of thrombolytic medicines are available but many of them have severe side effects which might lead to bleeding and embolism.54 In the previous studies, it was reported that secondary metabolites present in the plant extract might be responsible for the thrombolytic activity. Fuentes et al55 determined that flavonoids and saponins are the phytochemicals responsible for the thrombolytic activity of plants and these compounds have ability to dissolve the fibrin clot.
In Vivo Hydroethanolic Extract’ Therapeutic Response
Hepatoprotective and renoprotective activities of E. grandiflorum extract
Liver as a chief organ in body plays vital roles in the body like synthetic, detoxification, storage, excretion as well as secretion; and the failure of any these metabolic process might associated with liver abnormalities leading to increased serum liver enzymes levels in blood circulation.56 The improvement in liver enzymes of plants treated rats suggests the extract has hepatoprotective potential of Epimedium.2 It was also found that Epimedium species have potential to restore the transaminases reported by Lee et al.57
In Chinese Traditional Medicine, the kidney is alleged to be crucial for sexual and bone health. To tonify the renal functions and stimulate the yang, epimedium species are under in clinical practice and latest in vitro and in vivo pharmacological studies demonstrated that epimedium plants have a wide range of therapeutic applications including renoprotective activities.1 Intragastric administration of 5 g/kg b.w daily of Epimedium to male rats, it was reported that kidney functions are restored by recovering the injured Kidney cell which also up regulate the bone formation by increasing the expression of renal hormone genes.58
Total oxidant (TOS), antioxidant status (TAS) and stress markers in rats’ blood
Antioxidant systems both enzymatic and non-enzymatic act the natural body defense mechanism to neutralize the oxidant molecules. Total antioxidant status in the body often revealed the dynamic equilibrium between the body’s antioxidant defense and pro-oxidants.59 Physiologically, body have capacity to generate enough antioxidants or are taken as food supplements for the detoxification of free radical species produced60 and in addition, oxygen free radicals to provide the energy source produced due to the enzymatic reduction of oxygen.61 Our results are coherent with the finding of Yang et al,62 who also reported the antioxidant potentials of Epimedium species in experimental rats. As in vitro analysis of E. grandiflorum for antioxidant parameters including reducing as well as scavenging potential revealed good antioxidant activities which might be due to the presence of high contents of flavonoids and phenolic compounds as their active secondary metabolites, therefore these compounds also provide good antioxidant defense system in animals after intoxication with CCl4.63
Change in body, testis weight, reproductive hormones and histo-architecture of testis
Epimedium plants not only used as medicines in Chinese Traditional medicine for the treatment of disease but also have importance to prevent disease and strengthen the body as supplement.1 Reproductive hormones including gonadotrophins, testosterone and prolactin influence erectile processes in males.64,65 The reproduction is tightly regulated by hypothalamo-pituitary gonadal axis and Gonadotrophin releasing hormone (GnRH) from hypothalamus influence the secretion of gonadotrophins; FSH and LH from the anterior pituitary. FSH stimulate the spermatogenesis while LH have role in the production and release of testosterone, male reproductive hormone, from the leydig cells essential for spermatogenesis and for reproductive system development like testis and prostate.66 It also regulate the expression of secondary sexual characteristic in males like increased muscle mass, bone mass, the endocrine system and growth of body-hair.67 FSH function is to stimulate the process of spermatogenesis while LH play role in stimulating the testosterone synthesis and release. Increased blood flow is influenced by testosterone which in turn stimulates the growth of the target tissues. The current study is in agreement with findings of Khan and Ahmed,68 who also reported that CCl4 induced testicular toxicity in rats and described that administration of CCl4 caused the atrophy of testicular tissue, germinal layer degeneration, lowered the serum level of gonadotropins (FSH and LH) and testosterone in male rat.68 The impairment in reproductive hormones might be due to the direct impact of CCl4 on leydig cells and make them unresponsive to FSH and LH, leads to decreased secretion of testosterone. In addition, it was also reported that many hormonal fluctuations like lower level of serum cortisol, testosterone, FSH, and insulin and impairment of prolactin secretion are associated with liver disorders in males.63,65 Moreover, it could be suggested that CYP17A1 enzyme action is inhibited by the action of CCl4 during intoxication phase and in the leydig cells progesterone produced could not be metabolized to androgen which ultimately leads the decreased concentration of testosterone and increased level of progesterone. Hence the decreased level of progesterone and increased level of testosterone in treated animal groups revealed the effects of plants extract are to counter this inhibitory effect of CCl4.69
Moreover, it was also find that Epimedium extracts possess a male-hormone-like effect and the administration of epimedium extract (2.0 mg/kg body weight (bw) for 2 week (14 days) orally daily significantly increased the sexual function, weight of attached genitals and improved the testosterone level in the plasma in rats.70 Furthermore, Luo,71 investigated that glycosides from E. grandiflorum 5 g/kg for 8 weeks to guinea pigs significantly improved the body weight, testicular growth, spermatozoa parameters including density, viability in the epididymis and vice-testis and also increased the of the testicles, epididymis, adenohypophysis and seminal vesicles. The improvement in the sexual arousal was also reported on administration of plant extract that might be due to improvement in the reproductive hormones.71 Our finding are also coherent in the findings of She et al., who on administration of the total flavonoids from E. brevicornum (150.0 mg/kg bw for 7 days) to the rats, observed significantly raised the level of reproductive hormones including testosterone, estradiol and luteinizing hormone levels as well as increased the weight of the anterior pituitary gland, epididymis and seminal vesicles in juvenile rats. Such finding revealed the Epimedium plants improving impact on both structural male reproductive system and reproductive endocrine activities.72
It could be concluded that E. grandiflorum contained a wide range of phytochemical constituents, their significant in vitro and in vivo antioxidant potential, significant hepatoprotective, renoprotective and reproductive hormones restoring capacity in male albino rats as therapeutic response directly proportional to doses of hydroethanolic extract administered. However, more research is required to isolate the novel compounds from this therapeutic plant to address the problems particularly of impotency.
Acknowledgments
The authors acknowledged the technical supervision and providing the space in Clinical Biochemistry Laboratory, Department of Biochemistry, Government College University Faisalabad–Pakistan to complete this research work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Naveed Munir  https://orcid.org/0000-0003-0380-1332
https://orcid.org/0000-0003-0380-1332
References
- 1. Ma H, He X, Yang Y, Li M, Hao D, Jia Z. The genus Epimedium: an ethnopharmacological and phytochemical review. J Ethnopharmacol. 2011;134(3):519–541. [DOI] [PubMed] [Google Scholar]
- 2. Shindel AW, Xin ZC, Lin G, et al. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo. J Sex Med. 2010;7(1):1518–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ning H, Xin ZC, Lin G, Banie L, Lue TF, Lin CS. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology. 2006;68(6):1350–1354. [DOI] [PubMed] [Google Scholar]
- 4. Zhang J, Li AM, Liu BX, et al. Effect of icarisid II on diabetic rats with erectile dysfunction and its potential mechanism via assessment of AGEs, autophagy, mTOR and the NO–cGMP pathway. Asian J Androl. 2013;15(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang ZB, Yang QT. The testosterone mimetic properties of icariin. Asian J Androl. 2006;8(5):601–605. [DOI] [PubMed] [Google Scholar]
- 6. Nayernia K, Li M, Jaroszynski L, et al. Stem cell based therapeutical approach of male infertility by teratocarcinoma derived germ cells. Hum Mol Genet. 2004;13(14):1451–1460. [DOI] [PubMed] [Google Scholar]
- 7. Tournaye HJ, Cohlen BJ. Management of male-factor infertility. Best Pract Res Cl Ob. 2012;26(6):769–775. [DOI] [PubMed] [Google Scholar]
- 8. Speroff L, Fritz MA. (eds). (2005). Clinical Gynecologic Endocrinology and Infertility . Lippincott Williams & Wilkins. [Google Scholar]
- 9. Toshimori K, Ito C, Maekawa M, Toyama Y, Suzuki-Toyota F, Saxena DK. Impairment of spermatogenesis leading to infertility. Anat Sci Int. 2004;79(3):101–111. [DOI] [PubMed] [Google Scholar]
- 10. Austin DF. Ipomoea littoralis (Convolvulaceae)—taxonomy, distribution, and ethnobotany. Econ Bot. 1991;45(2), 251–256. [Google Scholar]
- 11. Ahmad M, Sultana S, Fazl-i-Hadi S, et al. An ethnobotanical study of Medicinal Plants in high mountainous region of Chail valley (District Swat-Pakistan). J Ethnobiol Ethnomed. 2014;10(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antunes AE, Liserre AM, Coelho AL, et al. Acerola nectar with added microencapsulated probiotic. Lwt-Food Sci Technol. 2013;54(1):125–131. [Google Scholar]
- 13. Aggarwal H, Ghosh J, Rao A, Chhokar V. Evaluation of root and leaf extracts of Glycrriza glabra for antimicrobial activity. J Med Biol Eng. 2015;4(1). [Google Scholar]
- 14. Taid TC, Rajkhowa RC, Kalita JC. A study on the medicinal plants used by the local traditional healers of Dhemaji district, Assam, India for curing reproductive health related disorders. Adv Appl Sci Res. 2014;5(1):296–301. [Google Scholar]
- 15. Esmaeili HR, Coad BW, Gholamifard A, Nazari N, Teimory A. Annotated checklist of the freshwater fishes of Iran. Zoosyst Ross. 2010;19(2):361–386. [Google Scholar]
- 16. Jain S, Jain A, Vaidya A, Kumar D, Jain V. Preliminary phytochemical, pharmacognostical and physico-chemical evaluation of Cedrus deodara heartwood. J Pharmacogn Phytochem. 2014;3(1). [Google Scholar]
- 17. Pranuthi EK, Narendra K, Swathi J, et al. Qualitative assessment of bioactive compounds from a very rare medicinal plant Ficus dalhousiae Miq. J Pharmacogn Phytochem. 2014;3(1). [Google Scholar]
- 18. Yadav RN, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011;3(12):10–14. [Google Scholar]
- 19. Ayaz M, Junaid M, Ahmed J, et al. Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from Polygonum hydropiper L. Bmc Complem Altern M. 2014;14(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yue X, Hao Y, Jian-Fei W, et al. Simultaneous determination of nine phenolic acids in dendranthema morifolium (Ramat) Tzvel. cv. Chuju samples by high performance liquid chromatography. Chin J Anal Chem. 2013;41(3):383–388. [Google Scholar]
- 21. Prieto P, Pineda M, Anguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a P hosphomolybdenum C omplex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269(2):337–341. [DOI] [PubMed] [Google Scholar]
- 22. Shahid Chatha SA, Hussain AI, Asad R, Majeed M, Aslam N. Bioactive components and antioxidant properties of Terminalia arjuna L. extracts. J Food Process Technol. 2014;5(2):298. [Google Scholar]
- 23. Yadav M, Yadav A, Yadav JP. In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac J Trop Med. 2014;7:256–261. [DOI] [PubMed] [Google Scholar]
- 24. Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–1008. [DOI] [PubMed] [Google Scholar]
- 25. Powell WA, Catranis CM, Maynard CA. Design of self-processing antimicrobial peptides for plant protection. Lett Appl Microbiol. 2000;31(2):163–168. [DOI] [PubMed] [Google Scholar]
- 26. Kumar G, Karthik L, Rao KV. Hemolytic activity of Indian medicinal plants towards human erythrocytes: an in vitro study. Elixir Applied Botany. 2011;40:5534–5537. [Google Scholar]
- 27. Rahman MA, Sultana R, Emran TB, et al. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement Altern Med. 2013;13(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahreen S, Khan MR, Khan RA, Shah NA. Effect of Carissa opaca leaves extract on lipid peroxidation, antioxidant activity and reproductive hormones in male rats. Lipids Health Dis. 2013;12(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kakkar P, Das B, Viswanathan PN. A modified spectrophotomateric assay of superoxide dismutase. Indian J Biochem Bio. 1984;21:130–132. [PubMed] [Google Scholar]
- 30. Chance B, Maehly AC. Assay of catalase and peroxidases. Methods Enzymol 1955;11:764–775. [Google Scholar]
- 31. Bergmeyer HU, Horder M, Rej R. IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate and alanine aminotransferases. J Clin Chem Clin Biochem. 1986;24:481–495. [PubMed] [Google Scholar]
- 32. Thomas L. Clinical Laboratory Diagnostics. 1st ed TH-Books Verlagsgesellschaft; 1998;36–46. [Google Scholar]
- 33. Koller A, Kaplan LA, Pesce AJ. (1984). Total Serum Protein, Clinical Chemistry, Theory, Analysis and Correlation. Mosby Company; 1984:1316–1319. [Google Scholar]
- 34. Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry. 2nd ed Saunders; 1994:625–888, 928–1081, 1513–1568. [Google Scholar]
- 35. Swanson AF, Swartzentruber M, Nolen PA, Crawford K, Sine HE. Multicenter evaluation of the boehringer Mannheim compensated, rate-blanked creatinine/jaffe application on BM/Hitachi systems. Advances in clinical diagnostics. Boehringer Mannheim Corporation. 1993:3–11. [Google Scholar]
- 36. Chen A, Bookstein JJ, Meldrum DR. Diagnosis of a testosterone-secreting adrenal adenoma by selective venous catheterization. Fertil Steril. 1991;55:1202–1203. [DOI] [PubMed] [Google Scholar]
- 37. Frank JE, Faix JE, Hermos RJ, et al. Thyroid function in very low birth weight infants: effects on neonatal hypothyroidism screening. J Pediatr. 1996;128(4):548–554. [DOI] [PubMed] [Google Scholar]
- 38. Qiu Q, Kuo A, Todd H, et al. Enzyme immunoassay method for total urinary follicle-stimulating hormone (FSH) beta subunit and its application for measurement of total urinary FSH. Fertil Steril. 1998;69(2):278–285. [DOI] [PubMed] [Google Scholar]
- 39. Vanderpump MP, French JM, Appleton D, Tunbridge WM, Kendall-Taylor P. The prevalence of hyperprolactinaemia and association with markers of autoimmune thyroid disease in survivors of the Whickham Survey cohort. Clin Endocrinol (Oxf). 1998; 48(1):39–44. [DOI] [PubMed] [Google Scholar]
- 40. Radwanska E, Frankenberg J, Allen EI. Plasma progesterone levels in normal and abnormal early human pregnancy. Fertil Steril. 1978;30(4):398–402. [PubMed] [Google Scholar]
- 41. Tsang BK, Armstrong DT, Whitfield JF. Steroid biosynthesis by isolated human ovarian follicular cells in vitro. J Clin Endocrinol Metab. 1980;51(6):1407–1411. [DOI] [PubMed] [Google Scholar]
- 42. Bancroft JD, Gamble M. Theory and practice of histological techniques. Elsevier Health Sci. 2008;625–725. [Google Scholar]
- 43. Montgomery DC. Design and Analysis of Experiments. 7th ed John Wiley & Sons; 2009. [Google Scholar]
- 44. Fowles RG, Mootoo BS, Ramsewak RS, Khan A. Toxicity–structure activity evaluation of limonoids from Swietenia species on Artemia salina. Pharma Biol. 2012;50(2):264–267. [DOI] [PubMed] [Google Scholar]
- 45. Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4(7):685–688. [Google Scholar]
- 46. Okwu DE, Okwu ME. Chemical composition of Spondias mombin Linn Plant parts. J Sustain Agric Environ. 2004;6(2):140–147. [Google Scholar]
- 47. Zheng X, Liu B, Li L, Zhu X. Micro-wave-assisted extraction and antioxidant activity of total phenolic compounds from pomegranate peel. J Med Plants Res. 2011;5(6):1004–1011. [Google Scholar]
- 48. Leake JR. Is diversity of ectomycorrhizal fungi important for ecosystem function?. New Phytol. 2001;152(1):1–3. [DOI] [PubMed] [Google Scholar]
- 49. Smolskaitė L, Venskutonis PR, Talou T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT-Food Sci Technol. 2015;60:462–471. [Google Scholar]
- 50. Jayakumar R, Chennazhi KP, Srinivasan S, Nair SV, Furuike T, Tamura H. Chitin scaffolds in tissue engineering. Int J Mol Sci. 2011;12:1876–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang J, Zhang HF, Cao XY, et al. Enzymatic water extraction of polysaccharides from Epimedium brevicornu and their antioxidant activity and protective effect against DNA damage. J Food Biochem. 2017;41(1):e12298. [Google Scholar]
- 52. Zhao WH, Deng ZY, Fan QS. Anti-oxidation on total flavones of Epimedium in vitro. J Nanchang Univ(Natural Science). 2009;30:53–60. [Google Scholar]
- 53. Zhao Y, Hou Y, Tang G, et al. Optimization of ultrasonic extraction of phenolic compounds from Epimedium brevicornum maxim using response surface methodology and evaluation of its antioxidant activities in vitro. J Anal Methods Chem. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Capstick T, Henry M. Efficacy of thrombolytic agents in the treatment of pulmonary embolism. Eur Respir J. 2005;26(5):864–874. [DOI] [PubMed] [Google Scholar]
- 55. Fuentes E, Guzmán L, Alarcón M, Moore R, Palomo I. Thrombolytic/fibrinolytic mechanism of natural products. Fibrinolysis Thrombolysis. 2014:107–121. [Google Scholar]
- 56. Nweze CC, Mustapha AA, Alkali IM. Aqueous leaf extracts of Tobacco plant (Nicotiana tabaccum) causes hepatotoxicity in male Wistar albino rats. Asian J Pharmacol Toxicol. 2015;3(07):27–30. [Google Scholar]
- 57. Lee MK, Choi YJ, Sung SH, Shin DI, Kim JW, Kim YC. Antihepatotoxic activity of Icariin, a major constituent of Epimedium koreanum. Planta Medica. 1995;61:523–526. [DOI] [PubMed] [Google Scholar]
- 58. Zhou L, Cui L, Wu T. The effects of Epimedium on the expressions of renal and femoral bone morphogenetic protein-7 in male rats with kidney-YANG insufficiency. Chin J Osteoporosis. 2008;14:90–94. [Google Scholar]
- 59. Ghiselli A, Serafini M, Natella F, Scaccin C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radical Biol Med. 2000;29(11):1106–1114. [DOI] [PubMed] [Google Scholar]
- 60. Castillo CF, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138(4):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. [DOI] [PubMed] [Google Scholar]
- 62. Yang X, Zhang YH, Ding CF, Yan ZZ, Du J. Extract from Epimedium brevicornum Maxim. Against injury to function of human sperm membrane in vitro. Chin J Clin Pharmacol Therapeutics. 2007;12:663–667. [Google Scholar]
- 63. Riaz M, Mahmood Z, Saeed MUQ, Abbas M, Shahid M. Lipid peroxidation and antioxidant enzymes in relation to semen quality. Oxid Commun. 2016;39(4-I): 2990–2998. [Google Scholar]
- 64. Bansal A. Sexual dysfunction in hypertensive men, a critical review of the literature. Hypertension. 1988;12:1–10. [DOI] [PubMed] [Google Scholar]
- 65. Khan MR, Khan GN, Ahmed D. Evaluation of antioxidant and fertility effects of Digera muricata in male rats. Afr J Pharm Pharmacol. 2011;5(6):688–699. [Google Scholar]
- 66. Rudolph LM, Bentley GE, Calandra RS, et al. Peripheral and central mechanisms involved in the hormonal control of male and female reproduction. J Neuroendocrinol. 2016;28:doi:10.1111/jne.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reed JL, Famili I, Thiele I, Palsson BO. Towards multidimensional genome annotation. Nat Rev Genet. 2006;7(2):130–141. [DOI] [PubMed] [Google Scholar]
- 68. Khan MR, Ahmed D. Protective effects of Digera muricata (L.) mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem Toxicol. 2009;47(6):1393–1399. [DOI] [PubMed] [Google Scholar]
- 69. Almeida J, Conley AJ, Mathewson L, Ball BA. Expression of steroidogenic enzymes during equine testicular development. Reproduction. 2011;141(6):841–848. [DOI] [PubMed] [Google Scholar]
- 70. Wang F, Zheng Y, Xiao HB, Zhou M, Liu YX, Li GQ. Effect of administration of herba Epimedium at the optimal time levels on sexual hormones. J Tradit Chin Med. 2001;42:619–621. [Google Scholar]
- 71. Luo M. Effect of herba epimediim on growth of guinea pig. Chin WildvPlant Resources. 1998;1:38–39. [Google Scholar]
- 72. She BR, Qin DN, Wang Z, She YC. Effeets of flavonoids from Herba Epimediim on the reproduetive system in male rats. Chin J Androl. 2003;17:294–296. [Google Scholar]