Fig. 10.
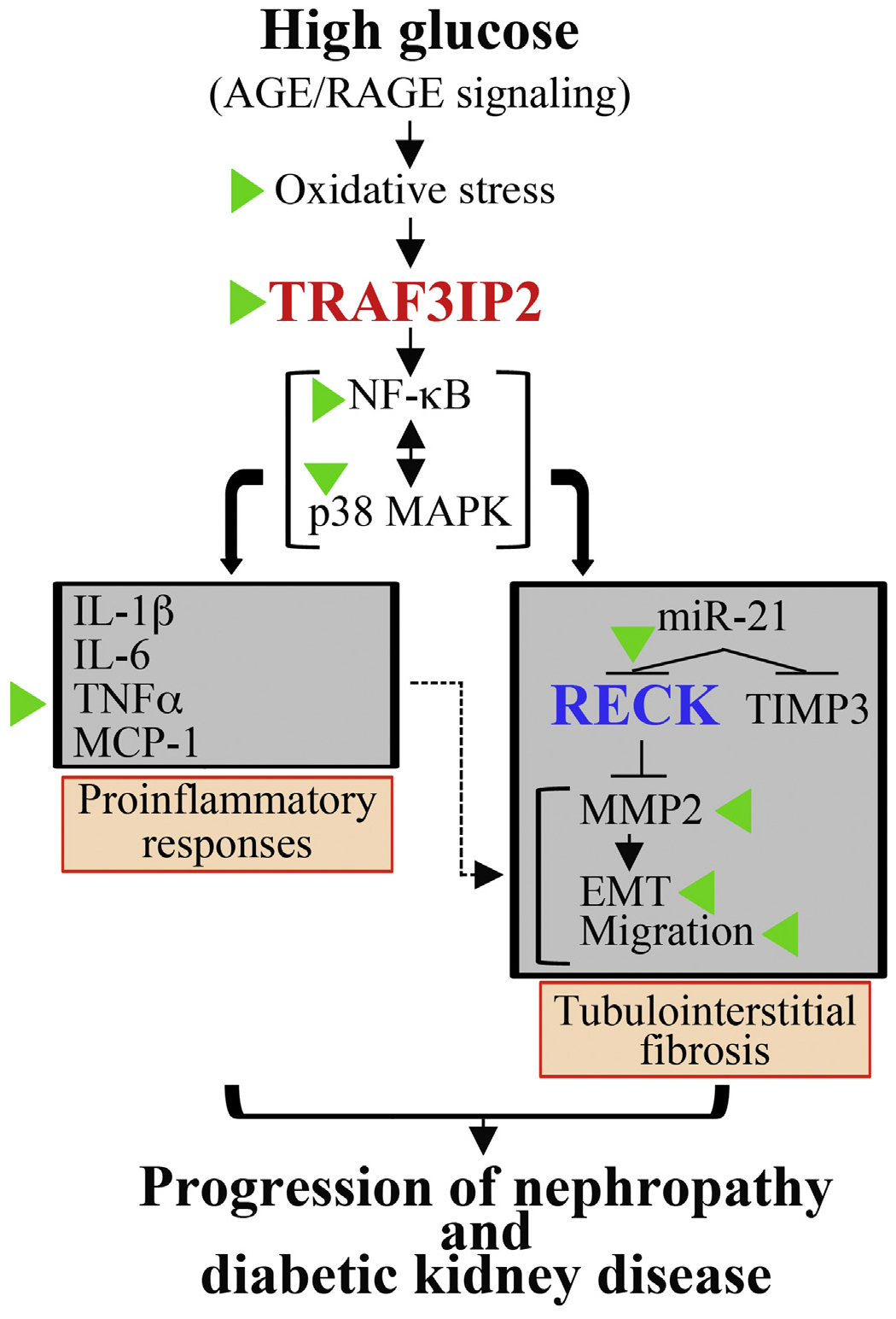
Schema showing the signal transduction pathway involved in high glucose (HG)-induced HK-2 cell proinflammatory response, epithelial-to-mesenchymal transition (EMT) and migration, and those molecules or events targeted by EMPA (indicated by green arrowheads). HG and AGE/RAGE induce oxidative stress as evidenced by increased generation of superoxide and hydrogen peroxide, oxidative stress-dependent TRAF3IP2 upregulation, and TRAF3IP2-dependent NF-κB and p38 MAPK activation, proinflammatory cytokine and miR-21 induction. Further, miR-21 suppresses RECK (and TIMP3) leading to activation of MMP2 that promotes EMT and cell migration that is further exacerbated by the proinflammatory response (indicated by a dashed line). We hypothesize that the illustrated signal transduction pathway leading to high glucose-induced oxidative stress, TRAF3IP2 induction, inflammation, MMP activation and RECK suppression contribute, in part, to progression of diabetic kidney disease. Similar to HG, its metabolite AGE also induces TRAF3IP2, but suppresses RECK. Importantly, EMPA has therapeutic potential in diabetic kidney disease by preventing HG and AGE-induced TRAF3IP2 expression and RECK suppression.
