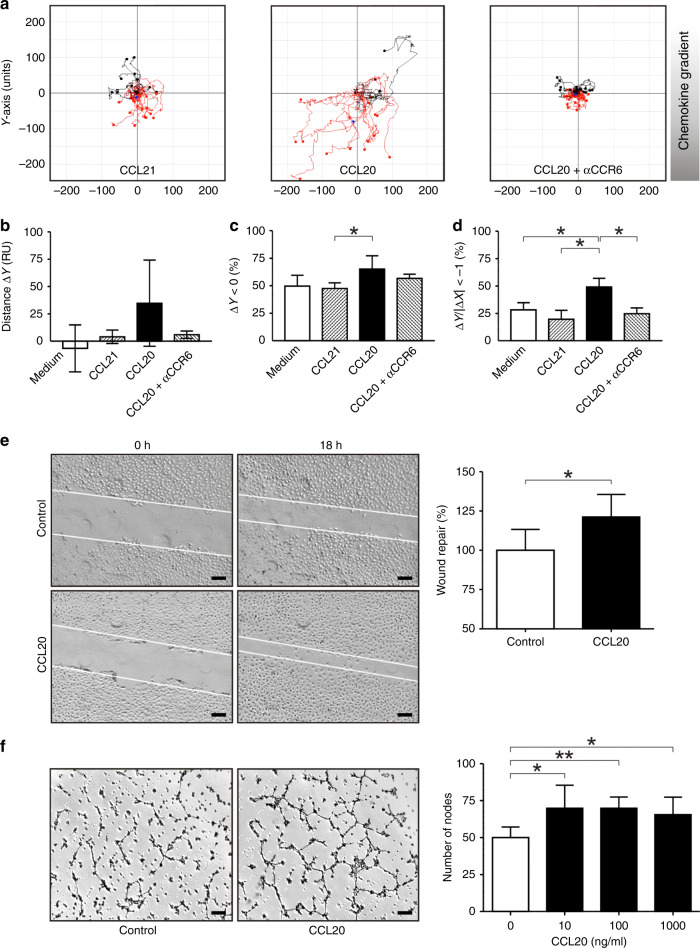
Fig. 4. CCL20 mediates directional migration of human microvascular endothelial cells and enhances tube formation.
a Representative trajectories of microvascular endothelial cells cultured inside an IBIDI µ-chemotaxis chamber containing CCL20 (1000 ng/ml), CCL21 (1000 ng/ml; “irrelevant” (the corresponding receptor is absent on the target cell) chemokine control) or endothelial cells pre-incubated with anti-CCR6 antibodies and cultured in slides containing CCL20 (1000 ng/ml). Trajectories are representatives for test and control stimuli of at least three different experiments. b–d Statistical analysis of endothelial cell trajectories in chemotaxis chambers. b Average ∆Y, mean net distance (RU) travelled along the chemokine gradient (Y axis). c ∆Y < 0, percentages of endothelial cells travelling in the direction of chemokine gradients (Y axis). (d) ∆Y/I∆XI < −1, percentages of endothelial cells travelling a longer distance in the direction of chemokine gradients (Y axis) than in the direction orthogonal to the gradients (X axis) (*P ≤ 0.05, Mann–Whitney U test). e In monolayer wound-repair assays, CCL20 was able to induce significant wound repair and migration of microvascular endothelial cells when compared with corresponding controls (*P ≤ 0.05, Mann–Whitney U test) [scale bars represent 200 µm]. f Tube-formation assay with microvascular endothelial cells showing representative images of endothelial cell tube formation on Matrigel alone or Matrigel supplemented with CCL20 (10 ng/ml), and statistical analysis of tube-formation assays. Number of nodes per field are shown and represent the mean ± SD of three independent experiments (*P ≤ 0.05; **P ≤ 0.01, Mann–Whitney U test) [scale bars represent 200 µm].