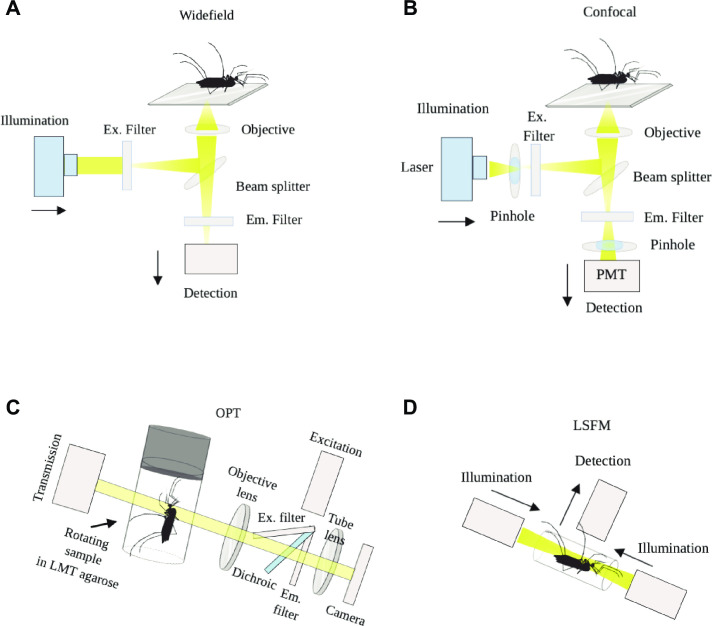
Fig 1. Microscopy methods used for imaging mosquitoes.
A) ‘Inverted’ widefield microscopy: White light is filtered to the appropriate emission wavelength, and the emitted fluorescent light is projected onto a camera. B) Confocal microscopy: laser light is focused onto the specimen and a pinhole excludes out of focus light. Instead of a camera, photomultiplier tubes (PMTs) collect photons. C) Optical projection tomography: The optically cleared specimen is embedded in agarose, attached to a metallic cylinder within a rotating stage, and suspended in an index-matching liquid to reduce scattering and heterogeneities of refractive index throughout the specimen. Images are captured at distinct positions as the specimen is rotated. The axis of rotation is perpendicular to the optical axis, so that straight line projections going through the sample can be generated, and collected on the camera. D) Light sheet fluorescence microscopy: The sample is embedded in agarose, and suspended within a sample holder inside an index-matching liquid. A thin (μm range) slice of the sample is illuminated perpendicularly to the direction of observation. Scanning is performed using a plane of light, which allows very fast image acquisition. This figure was created using BioRender.com. Diagrams to generate this figure are republished from BioRender under a CC BY license, with permission from BioRender, original copyright (2020).