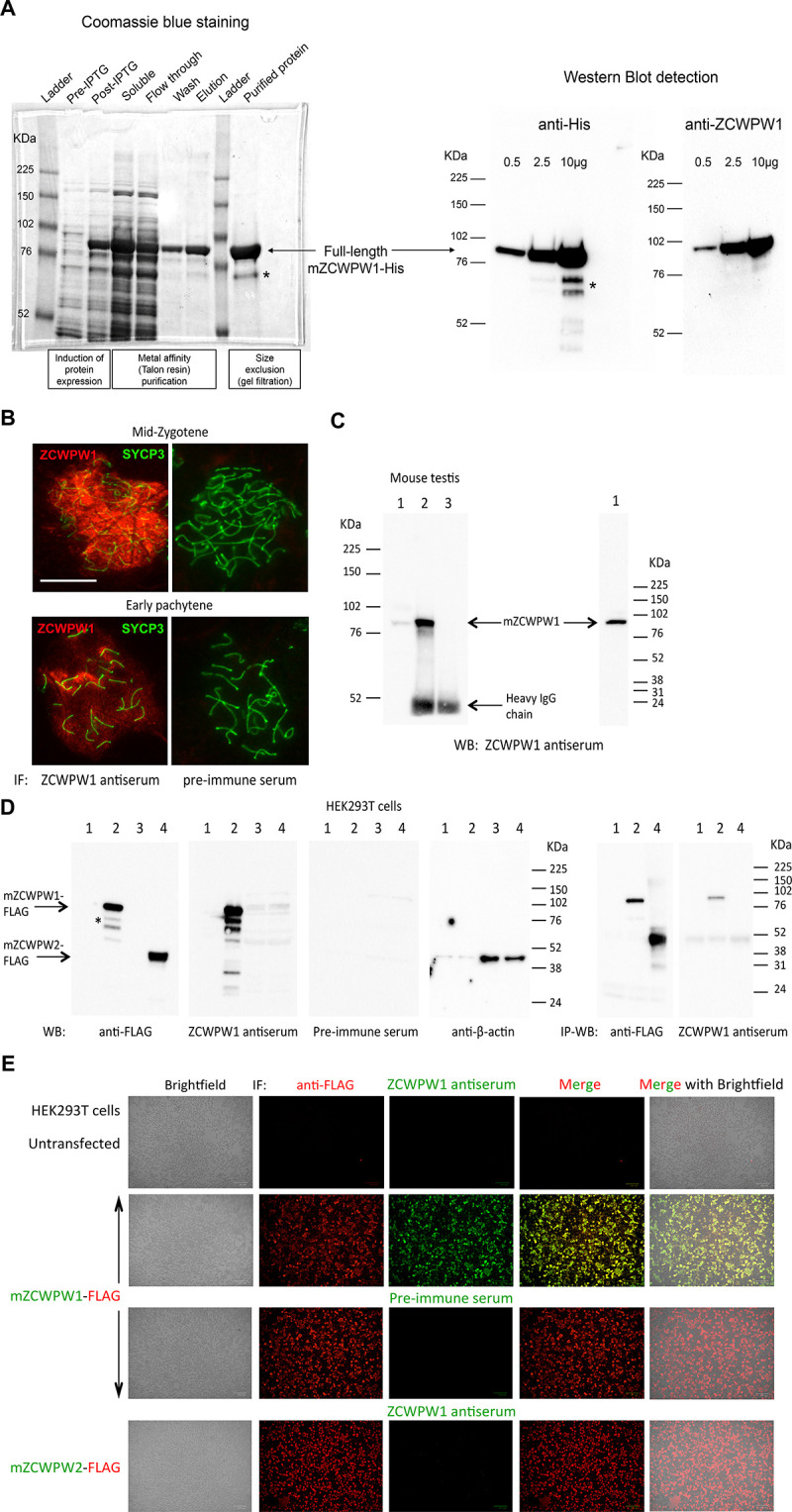
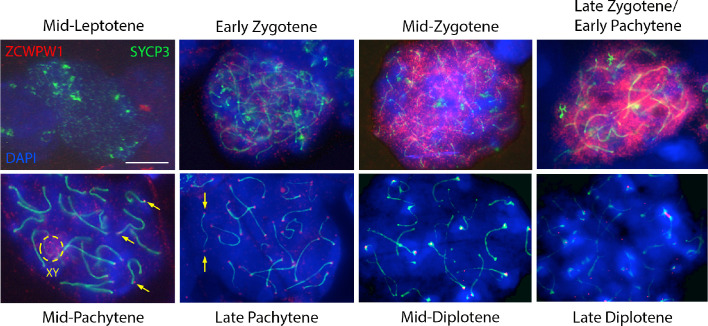
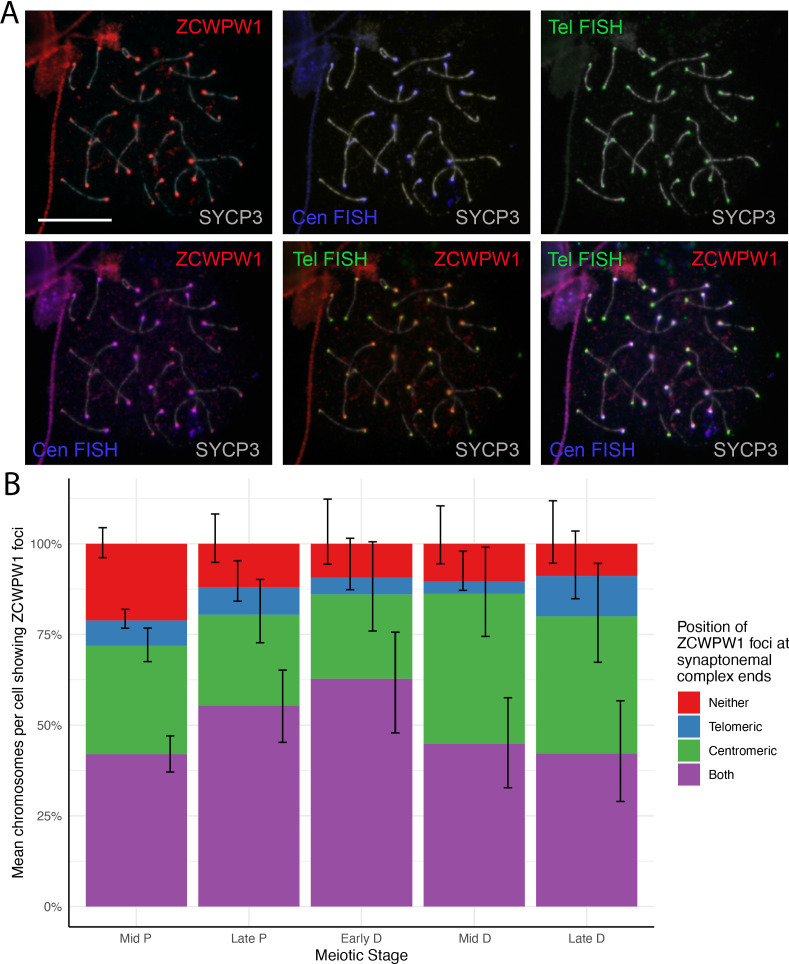
(A) Expression and purification of full-length recombinant mouse ZCWPW1 (mZCWPW1) in E. Coli. Left panel: SDS-PAGE analysis and Coomassie blue staining of bacterial lysates before (pre-IPTG) and after (post-IPTG) induction of protein expression with IPTG, the soluble protein fraction (after cell sonication) used for purification, the flow through (incompletely depleted from the target protein) after incubating the soluble fraction with Talon resin beads to bind His-tagged mZCWPW1, the wash containing 5mM imidazole, the protein eluate from the beads using 300mM imidazole, and the purified recombinant protein after further purification from low molecular weight (MW) contaminants by size exclusion. Right panel: Western blot detection of purified His-tagged mZCWPW1 using an anti-His and a mouse polyclonal antibody raised against the human protein (previously tested positively against mouse ZCWPW1 overexpressed in HEK293T cells). *Indicates degradation fragments (likely C-terminal). The purified protein was used to immunise rabbits and produce an antiserum against mZCWPW1. (B-E) Validation of the rabbit ZCWPW1 antiserum by immunofluorescence staining (IF), immunoprecipitation (IP) and western blotting (WB) in B6 testis (B,C) and transfected HEK293T cells (D,E). (B) Testis nuclear spreads from 10 weeks old B6 mice were immunostained with the ZCWPW1 antiserum or the pre-immune serum (red), and chromosome axes were labeled with SYCP3 (green). Representative mid-zygotene and early pachytene cells are shown. No signal is detected by the pre-immune serum. Scale bar: 10μm. (C) IP-WB detection of mZCWPW1 from 10 weeks old B6 mouse testis. Left panel: Lane 1, 100 µg protein extract; Lanes 2–3, IP from 2.6 mg protein extract using ZCWPW1 antiserum (lane 2) or the pre-immune serum (lane 3). The ZCWPW1 antibody detects a unique protein band within the expected MW range (predicted at 70.5 KDa) both by direct WB (lane 1) and IP-WB (lane 2). No signal is detected by the pre-immune serum (lane 3). Right panel: the testis protein extract was resolved on a higher (4–20%) SDS-PAGE gel; no detection of ZCWPW2 is observed by WB at the 38KDa MW range predicted for mouse ZCWPW2, only a single band is present within the expected MW range for ZCWPW1. The images in (B–C) are representative of the results obtained in two mice. (D) Specific IP-WB and WB detection of FLAG-tagged mZCWPW1 over ZCWPW2 from transfected HEK293T cells. Lanes 1,3: protein extracts from untransfected cells; Lanes 2,4: protein extracts from cells transfected with mZCWPW1-FLAG (lane 2) or mZCWPW2-FLAG (lane 4). Lanes 1–2, 5 µg extracts; lanes 3–4: 50 µg extracts. The ZCWPW1 antiserum detects the same protein band as the anti-FLAG antibody in the expected MW range (74 KDa) both by direct WB and by IP-WB, but does not show any reactivity against mZCWPW2-FLAG. The preimmune serum does not detect the mZCWPW1-FLAG protein band. Detection of beta-actin serves as a loading control. The asterisk indicates degradation fragments typically observed, as in (A). (E) Specific IF detection of FLAG-tagged mZCWPW1 over ZCWPW2 from transfected HEK293T cells. Cells were co-immunostained with ZCWPW1 antiserum or the pre-immune serum (green), and a FLAG antibody (red). The ZCWPW1 antiserum detects mZCWPW1-FLAG (precisely overlapping the signal detected by the FLAG antibody results in yellow fluorescence in the merged image), but not mZCWPW2-FLAG protein. No signal is detected with the pre-immune serum.