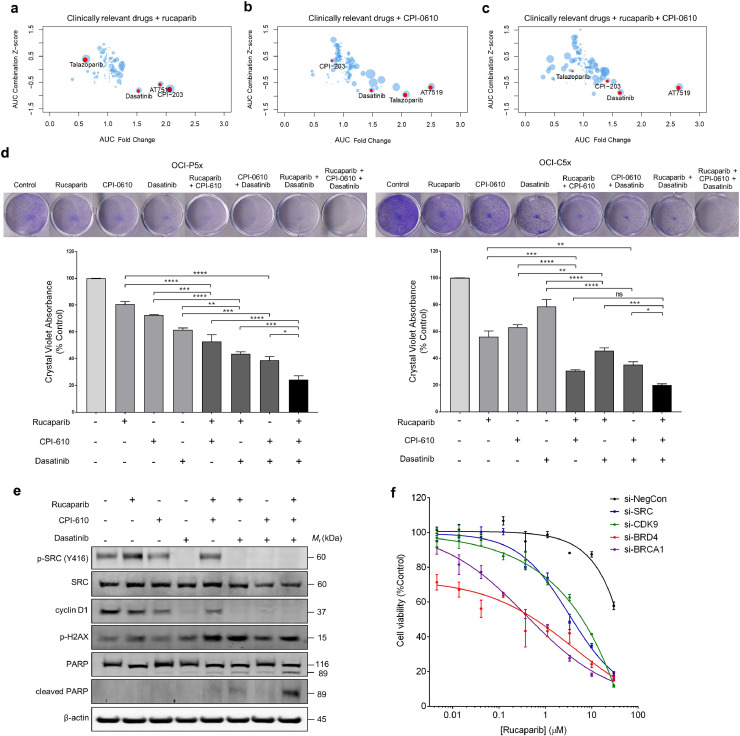
Fig. 3.
Dasatinib augments the combination of rucaparib and BETi. Drug screen utilising a 131 compound clinically focused library in combination with (a) rucaparib, (b) CPI-0610, and (c) rucaparib and CPI-0610. Depicted results are fold change vs. AUC Z-score of the single agent plus rucaparib. Highlighted in red in the dot plot are confirmatory drug hits and drug hits common within all combination arms. (d) Clonogenic assays performed in OCI-P5x and OCI-C5x cells using rucaparib (10 μM), CPI-0610 (1 μM), and dasatinib (100 nM) as single agents, in double combinations, or in triple combination. Quantitation of crystal violet staining is representative of at least three independent experiments. Data is presented as mean ± SEM: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns=not significant (one- way ANOVA followed by Tukey's test). (e) Western blot analysis of phospho-SRC (Tyr 416), SRC, cyclin D1, phospho-H2AX, PARP, and cleaved PARP in response to treatment with rucaparib (10 μM), CPI-0610 (3 μM), and dasatinib (300 nM) for 48 h in OCI-P5x cells. Blots shown are representative of at least three independent experiments. (f) OCI-P5x cells were treated with siRNA specific to SRC, CDK9, BRD4 or BRCA1 for 48 h. Cells were subsequently treated with rucaparib for a further 6 days before cell viability was measured. Representative of 3 independent experiments, data is presented as mean ± SEM.