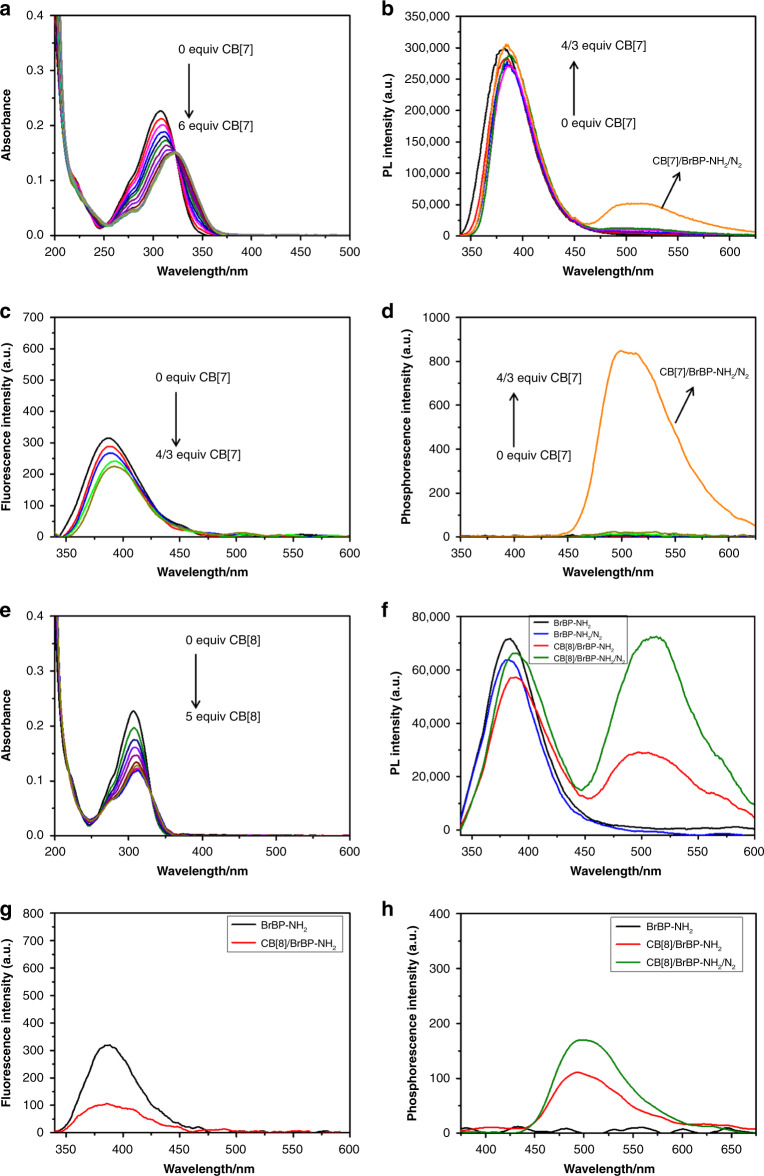
Fig. 2. Effect of complexation with CB[7] and CB[8] on the spectra of BrBP–NH2.
a Absorption spectra of BrBP–NH2 (0.01 mM) in the absence (black) and presence (yellow) of CB[7] (0.06 mM) in water at 25 °C. b Prompt photoluminescence spectra, c fluorescence spectra, and d phosphorescence spectra (delayed by 0.2 ms, Ex. Slit = 10 nm, Em. Slit = 10 nm) of BrBP–NH2 (black), CB[7]/BrBP–NH2 (yellow), and CB[7]/BrBP–NH2/N2 (orange) ([BrBP–NH2] = 0.5 mM, [CB[7]] = 0.67 mM) in water at 25 °C (λex = 320 nm). e Absorption spectra of BrBP–NH2 (0.01 mM) in the absence (black) and presence (red) of CB[8] (0.05 mM) in water at 25 °C. f Prompt photoluminescence spectra, g fluorescence spectra, and (h) phosphorescence spectra (delayed by 0.2 ms, Ex. Slit = 5 nm, Em. Slit = 5 nm) of BrBP–NH2 (black), CB[8]/BrBP–NH2 (red), and CB[8]/BrBP–NH2/N2 (green) ([BrBP–NH2] = 0.5 mM, [CB[8]] = 0.25 mM) in water at 25 °C (λex = 320 nm).