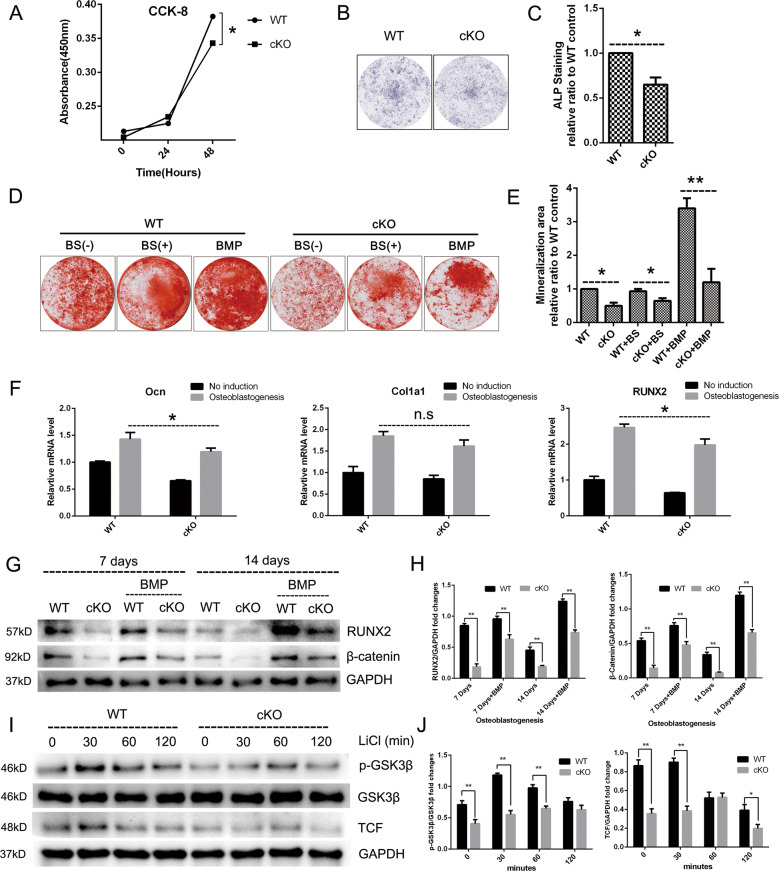
Fig. 6. Deletion of PKC-δ selectively in B cells led to impairment of osteoblast proliferation, differentiation, and function.
a Effect of PKC-δ cKO on osteoblast cell viability measured by the CCK-8 assay at day 1 and day 2. b, c Representative low-power images of alkaline phosphatase (ALP) staining (b) and quantitative analysis of ALP staining intensity relative to WT control group (c). d, e Representative low-power images showing the mineralized area stained with alizarin red (d) and quantitative analysis of the area of staining relative to WT control (e). f PKC-δ cKO suppressed osteoblast-specific genes transcription revealed by RT-PCR. Runx2, Ocn, and Col1a1 gene transcription level were tested after 7 days of osteogenesis induction. g, h Representative images of western blotting reflecting the expression levels of β-catenin (Wnt/β-catenin pathway) and RUNX-2 normalized to GAPDH after 7 and 14 days of induction with and without BMP-2 co-culture (g), and quantitative analysis of the fold changes (h). BMP-2 was used as a positive control. i, j PKC-δ cKO interacted with GSK-3β phosphorylation and TCF transcription. Representative western blotting images of p-GSK-3β, GSK-3β, TCF, and GAPDH at 0, 30, 60, and 120 min stimulated with 20 mM LiCl (i) and quantitative analysis of the fold changes of p-GSK-3β and TCF expression (j). BS B-cell supernatant, BMP bone morphogenetic protein-2 (25 ng/ml). All the experiments were carried out in triplicate from 6-week-old male WT and cKO mice, results are presented as mean ± SD. n.s. no statistical significance, *p < 0.05, **p < 0.01 vs. WT control group.