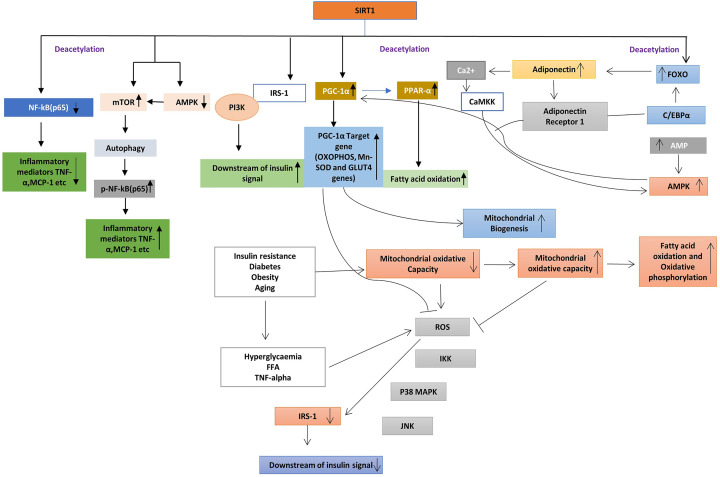
Figure 5. In adipocytes/macrophages, SIRT1 deacetylation leads to a decrease in the expression of several inflammatory mediators such as MCP-1 and TNF-α.
SIRT1 inactivation leads to NF-kB phosphorylation related to activation of mammalian target of rapamycin (mTOR) and decrease in expression of AMP-activated kinase (AMPK). In adipocytes, SIRT1 deacetylates the nuclear factor NF-kB p65, leads to a decrease in the expression of TNF-α and MCP-1. In skeletal muscle, SIRT1 induces the expression of peroxisome proliferator-activated receptor (PPAR-) co-activator 1α. Under diabetes condition mitochondrial oxidative capacity reduced which leads to the generation of free radical oxygen species, free fatty acids and TNFα reduced the insulin signaling via insulin receptor substrate phosphorylation. SIRT1 also activate phosphoinositide 3-kinase (PI3K). SIRT1 activates the PGC-1α induces the mitochondrial biogenesis which reduced the generation of ROS, increase the generation of GLUT4. SIRT1 deacetylates the forkhead box protein O1 (FOXO1) and increased the recruitment with CAAT/enhancer-binding protein (C/EBPα) which increase the generation of adiponectin1 in adipocytes. Adiponectin also activates the calcium/calmodulin-dependent protein kinase kinase (CaMKK) and calcium/calmodulin-dependent protein kinase kinase (CaMK). Adiponectin activates the SIRT1 via AMPK pathway activation. Its leads to deacetylation of PGC-1α resulted in mitochondrial biogenesis.