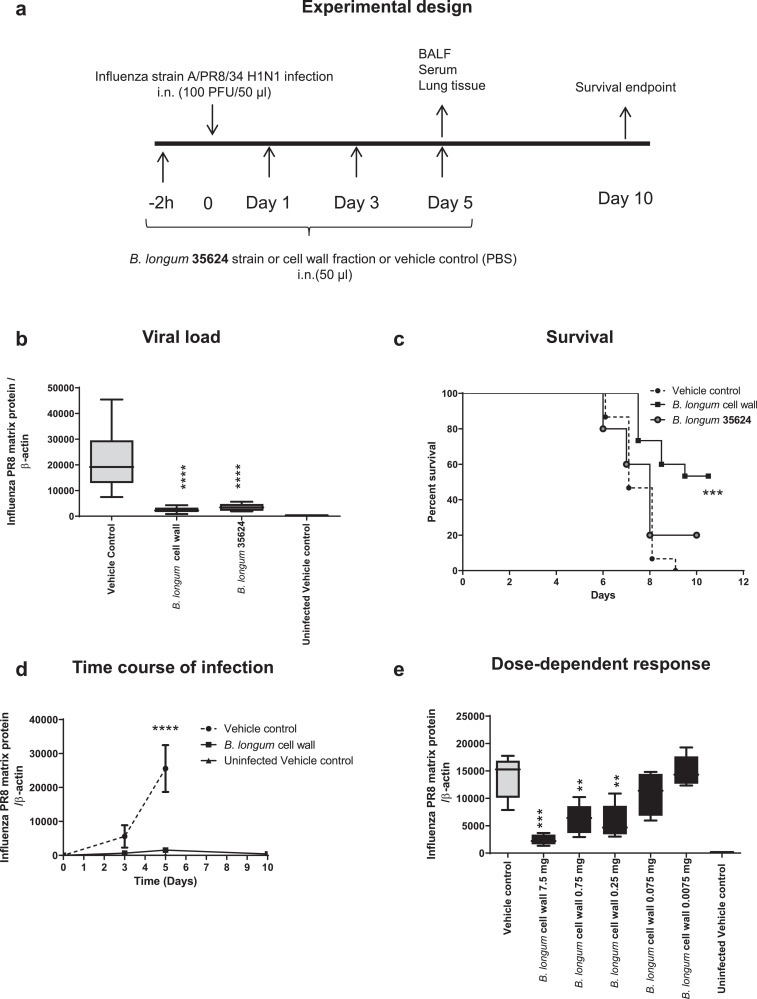
Fig. 1.
Intranasal B. longum35624®protects against lethal influenza infection.
(a) Experimental model outline for infection with 100 plaque forming units (PFU) of strain A/PR8/34 H1N1 at time 0, following intranasal administration of B. longum35624 bacterial cells, its isolated cell wall, or the vehicle control, at 2 h pre-infection and days 1, 3 and 5 post innoculation. (b) Viral load at day 5 post-innoculation in lung tissue as determined by quantitative real-time PCR (viral matrix protein normalized to β-actin expression). Data are presented as box-and-whisker plots with the median values and maximum/minimum values illustrated of 5 animals per group. ****p < 0.0001 (1-way ANOVA). (c) Survival was monitored up to 10 days post-innoculation (n = 5–15 animals per group). ***p < 0.001 (log-rank Mantel-Cox test). (d) Lung tissue viral load was determined at days 3, 5 and 10 post-innoculation. Data shown are mean ± SEM results of 5 animals per group for each time point. ****p < 0.0001 (2-way ANOVA). (e) Lung tissue viral load was determined at 5 days post-innoculation in animals administered different doses of isolated cell wall. Data are presented as box-and-whisker plots with the median values and maximum/minimum values illustrated of 5 animals per group. ***p < 0.001; **p < 0.01 (1-way ANOVA).