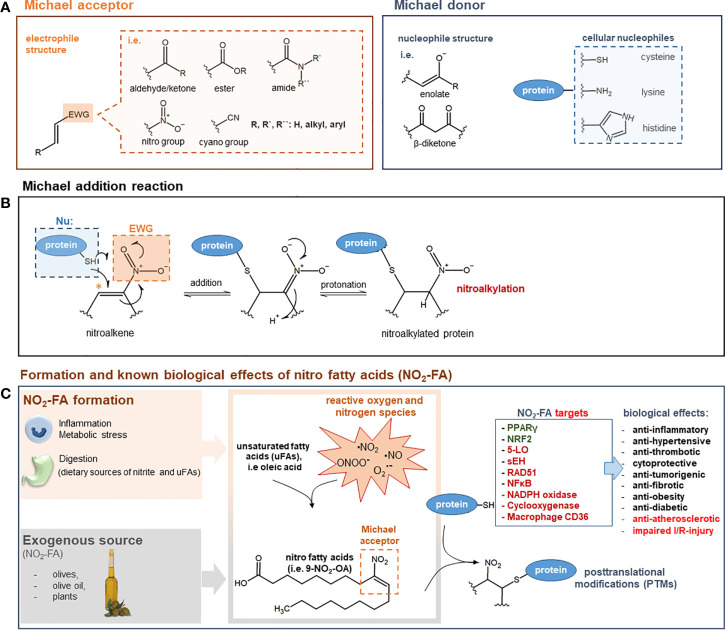
Figure 1.
(A) General structure of Michael acceptors and Michael donors. Michael acceptor moiety: electron withdrawing group (EWG) adjacent to an olefin structure forming an electrophilic, electron-deficient olefin. Examples of EWGs: aldehyde, keto, ester, amide, cyano, or nitro groups. Michael donor: nucleophiles such as enolates, β-diketones, thiols of cysteines, imidazoles of histidines, or ϵ-amino groups of lysines. (B) Mechanism of Michael addition reaction. The Michael addition reaction is exemplified by the attack of a cellular nucleophile to the electrophilic β-carbon (*) of a nitroalkene moiety. After the addition of the thiolate anion a protonation step takes place to form a nitroalkylated protein. (C) Formation and known biological effects of nitro fatty acids (NO2-FA). NO2-FA can be endogenously generated during inflammation by a reaction of nitric dioxide (NO2) with unsaturated fatty acids. Nitric dioxide can derive from different reactive nitrogen species (i.e nitric oxide, peroxynitrite) or precursor molecules like nitrate (NO3−) and nitrite (NO2−). NO2-FA can also be directly supplemented as natural ingredients of olives, olive oil and plants NO2-FA engage in cell signaling processes via the post-translational modification (PTM) of nucleophilic protein targets such as 5-LO, PPARγ, sEH, or NF-kB (proteins highlighted in green: activated/increased activity/expression; proteins highlighted in red: inhibited/decreased activity/expression). These PTMs induce profound changes in protein function and distribution and are therefore the leading cause for numerous biological effects. For a comprehensive overview on NFA targets and therapeutic effects see (Schopfer and Khoo, 2019). 5-LO: 5-lipoxygenase; NF-κB: nuclear factor-κB; NRF-2: nuclear factor erythroid 2-related factor 2; PPARγ: peroxisome proliferator–activated receptor γ; sEH; soluble epoxide hydrolase; I/R: ischemia/reperfusion.