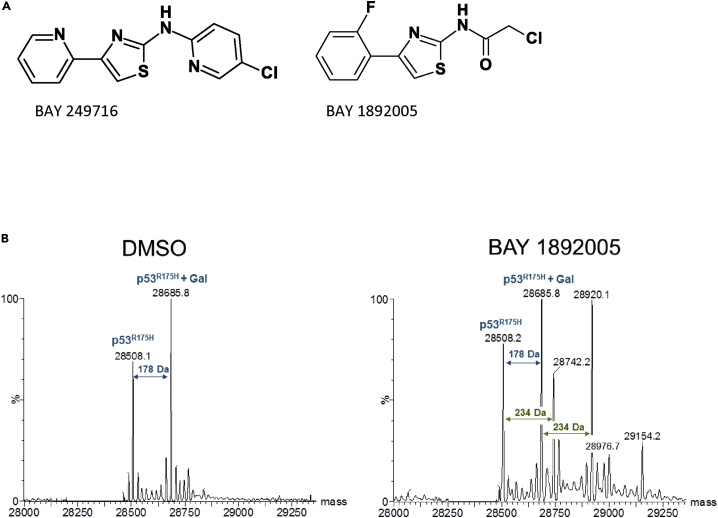
Figure 2.
Identification of Compounds that Interact with p53 Proteins
(A and B) (A) Hit compounds. (B) Deconvoluted spectra of intact mass analysis of p53 shows covalent binding of BAY 1892005 to p53R175H. DMSO control shows two peaks of p53R175H protein, one for the expected mass of 28,508 Da and one of 28,685 Da representing N-terminal gluconoylated (Gal) p53R175 (blue arrows). Incubation with BAY 1892005 showed mass shifts of 234 Da to both the apoprotein and the glyconoylated p53R175H (green arrows), indicative for covalent binding of BAY 1892005. An additional mass shift of 234 Da indicates a partial two-fold binding of BAY 1892005 to p53R175H. Exemplary data of multiple experiments are shown (n ≥ 2).