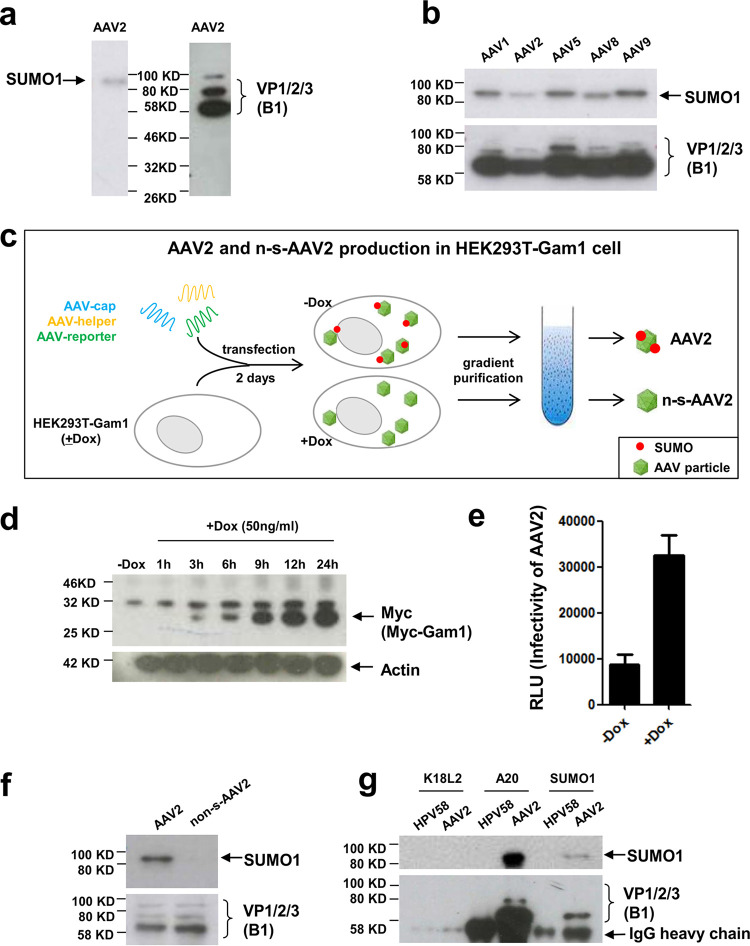
FIG 1.
AAV2 capsids are SUMOylated. Shown is an analysis of purified AAV2 particles. To determine AAV2 capsid modification by SUMO, we analyzed AAV2 vectors after purification by iodixanol gradient centrifugation. (a and b) AAV2 capsids and AAV1, -5, -8, and -9 capsids produced in 293T cells and analyzed by anti-SUMO1-specific antibodies or an antibody specific for all three AAV capsid proteins (B1 antibody). (c) Strategy to produce non-SUMOylated (n-s-) AAV2. HEK293T-Gam1 cells were transfected with three plasmids for AAV vector production. Induction of the avian adenovirus CELO Gam1 protein by doxycycline (Dox) leads to inactivation of the Sae1/2 E1 complex, leading to inactivation of the SUMOylation machinery. (d) Western blot analysis of Gam1 protein expression over time after Dox induction using Myc-tag antibody. HeLa-Gam1 cells were induced with 50 ng/ml Dox, and then cell lysate was harvested after 1, 3, 6, 9, 12, and 24 h. (e) Induction of Gam1 leads to higher transduction rates by AAV. HEK293T-Gam1 cells were incubated with and without Dox and then transduced with (SUMOylated) AAV-Luc vectors. RLU, relative light units. (f) Western blot analysis of AAV2 particles produced in 293T-Gam1 cells with (n-s-AAV2) or without Dox induction using SUMO1 and VP-specific antibodies. (g) Immunoprecipitation of AAV capsids. Purified AAV2 capsids were precipitated either with the AAV capsid-specific antibody A20 or with an anti-SUMO1 antibody. Precipitated proteins were then detected with either VP-specific antibody B1 or anti-SUMO1 antibody. As a specificity control, purified human papillomavirus type 58 capsids were precipitated using an unrelated antibody (K18L2).