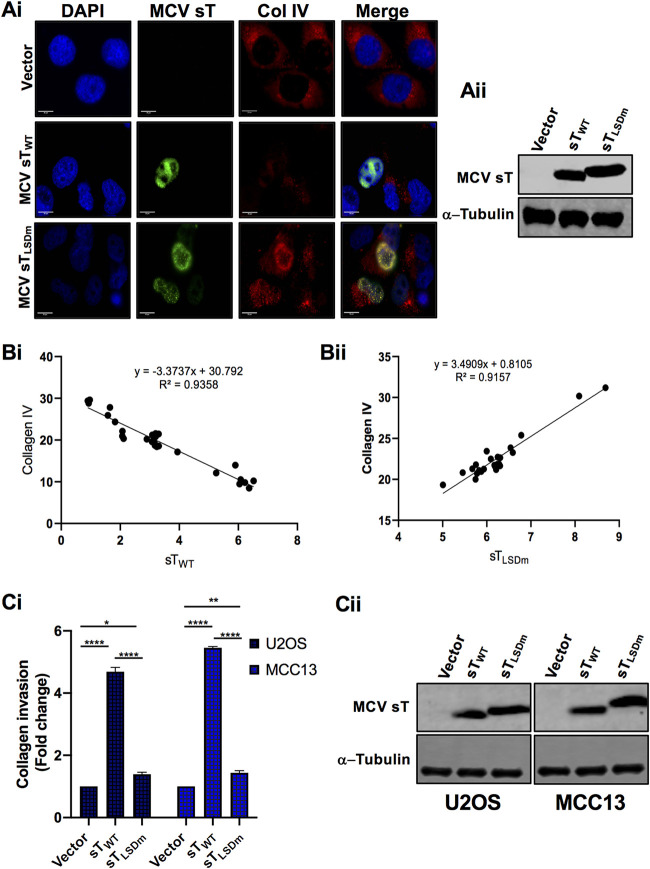
FIG 6.
MCV sT LSD induces collagen degradation. (A) MCV sT decreases collagen IV expression. U2OS cells were transfected with empty vector control, MCV sTWT, or MCV sTLSDm plasmids. (i) Cells were fixed at 48 h posttransfection, and endogenous collagen IV levels were measured by indirect immunofluorescence using a specific antibody. MCV sT expression was detected with 2T2 antigen antibody. Nuclear counterstain (4′,6-diamidino-2-phenylindole [DAPI]; blue), MCV sTWT and MCV sTLSDm (green), and collagen IV (red). (ii) Protein expression levels of wild-type and mutant MCV sT were detected by immunoblot analysis to validate successful transfection. Quantitative infrared fluorescence immunoblotting was performed using a 2T2 antibody for sT antigen and α-tubulin as an equal loading control. (B) MCV sT regulates collagen IV expression through the LSD. Mean fluorescence intensity of collagen IV in wild-type (i) and LSD mutant sT-expressing cells (ii) was analyzed using Fiji ImageJ software. The calculated values were plotted for regression analysis using Prism software. (C) MCV sT induces cell invasion through the LSD. (i) Collagen invasion assay. Serum-starved sT-expressing U2OS and MCC13 cells were seeded on the precoated collagen inserts and incubated for 72 h and then labeled with a cell-staining solution for 20 min. Upon extraction of the cell staining solution, absorbance at optical density at 560 nm (OD560) was measured. Data analyzed using three replicates per experiment; the experiments were performed two times. The results were reproducible, and differences between means (P value) were analyzed using a t test with GraphPad Prism software. (ii) Expression of MCV sT. Protein expression levels of wild-type and mutant MCV sT were detected by immunoblot analysis to validate successful transfection. Quantitative infrared fluorescence immunoblotting was performed using a 2T2 antibody for sT antigens and α-tubulin as an equal loading control.