FIG 5.
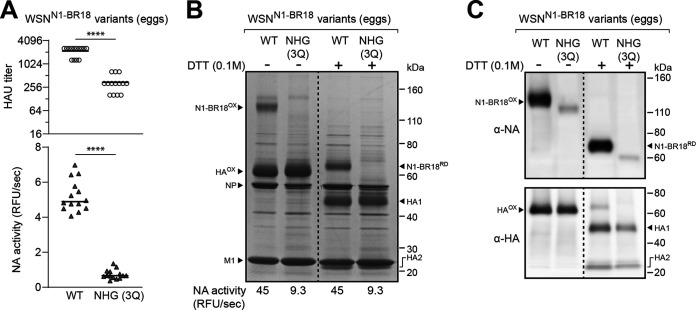
N1 viral incorporation is reduced when the conserved head glycosylation sites are absent. (A) Scatterplots of the HAU titers and NA activities in the allantoic fluid harvested from eggs infected with recombinant WSN viruses carrying N1-BR18 (WT) or N1-BR18 with no head glycan sites (NHG 3Q), which was generated by Gln mutations of each head glycosylation site (N88Q, N146Q, and N235Q). The viruses were rescued by reverse genetics and passaged twice in eggs. The data points from individual eggs following the second passage are shown with the median. P values (95% CI) are from a two-tailed unpaired t test. (B) Representative image of a Coomassie-stained SDS-PAGE gel containing the indicated recombinant WSNN1-BR18 viruses. The allantoic fluid from a second passage in eggs was pooled, the virions were isolated by centrifugation, and the protein concentration was determined. Samples containing ∼5 μg of total protein were treated with the reductant DTT as indicated and resolved using a 4–12% Tris-glycine SDS-PAGE gel. Intermolecular (N1-BR18OX) and intramolecular (HAOX) disulfide-bonded NA and HA are indicated along with the reduced forms (N1-BR18RD, HA1, and HA2). The viral proteins NP and M1 are also indicated. The NA activity listed below the gel was measured using equal total protein amounts of the two viruses. (C) NA and HA immunoblots of the isolated recombinant WSNN1-BR18 viruses. Samples containing equal total protein amounts were treated with DTT as indicated, resolved using a 4%–12% Tris-glycine SDS-PAGE gel, and transferred to a PVDF membrane prior to immunoblotting.