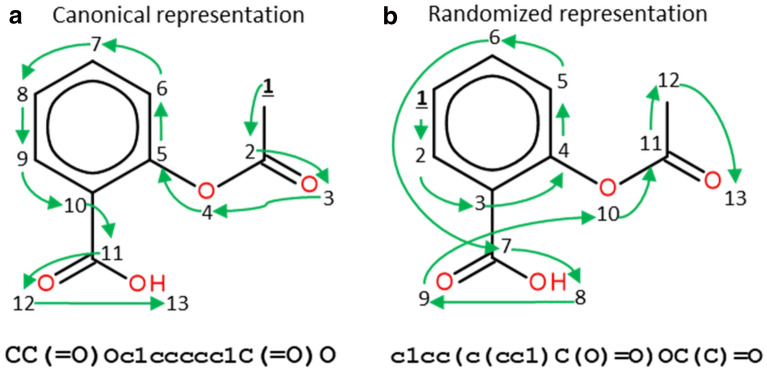
Fig. 4.
Canonical (a) and randomized (b) SMILES representations of aspirin. Randomized SMILES correspond to the various representations of a molecule obtained by randomly selecting the starting node in the graph traversal algorithm, thus changing the order of the nodes traversed in the molecular graph (still using depth-first search). Numbers represent the order of graph traversal, where 1 is the initial node (user defined). Considering a as being the canonical representation of aspirin, b shows a different ordering of the atoms of the molecule. The final SMILES is one possible SMILES among all the randomized SMILES which can be generated. Green arrows indicate how the molecular graph is traversed. Both SMILES strings shown represent the same molecule but, as the atom numberings are different, the generated SMILES strings are, too. The original figure can be found in [47]