Table 3.
Evidence of systemic treatments for psoriasis in different clinical conditions
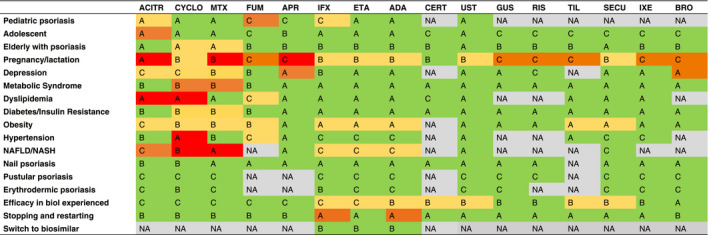
Levels of evidence: A (high level of evidence), B (moderate level of evidence), C (low level of evidence).
Results of the studies: 1. Green: preserved efficacy without increased adverse events or worsening of the comorbidity; 2. Yellow: limited risk of decreased efficacy and/or limited risk of increased adverse events or worsening of the comorbidity, 3. Orange: moderate risk of decreased efficacy and/or moderate risk of increased adverse events or worsening of the comorbidity, 4. Red: important risk of decreased efficacy and/or moderate risk of increased adverse events or worsening of the comorbidity.
ACIT, acitretin; ADA, adalimumab; APR, apremilast; BROD, brodalumab; CERT, certolizumab pegol; CYCLO, cyclosporin; ETA, etanercept; GUS, guselkumab; IFX, infliximab; IXE, ixekizumab; RIS, risankizumab; SEC, secukinumab; TIL, tildrakizumab; UST, ustekinumab.
