Figure 4.
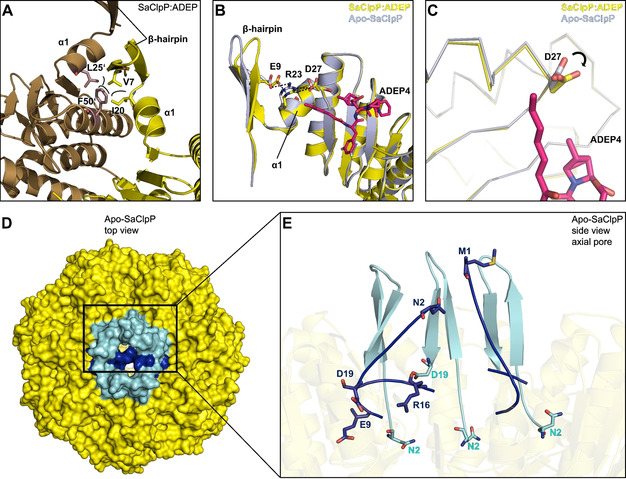
Crystal structures of SaClpP in its apo (PDB ID: 6TTY) and ADEP4‐bound (PDB ID: 6TTZ) forms with focus on the N‐terminal cluster. A) Inter‐subunit connection: depiction of N‐terminal hydrophobic interactions mediating inter‐subunit stability and β‐hairpin orientation. Two adjacent ClpP subunits are depicted in yellow and brown. B) Intra‐subunit connection: Overlay of the N‐terminus of SaClpP:ADEP4 (yellow) with the N terminus apo‐SaClpP in a β‐hairpin orientation (grey). Key residues of the N‐terminal hydrogen‐bonding network display a shift in orientation that propagates to the axial pore. C) Rotation of the D27 residue upon binding of ADEP4. D) Top view of the apo‐SaClpP crystal in a surface representation. N‐terminal β‐hairpin regions (residues 1–19) that adopt the “up conformation” are coloured cyan, corresponding regions that adopt the “down conformation” are coloured dark blue. E) N‐terminal β‐hairpin regions of five ClpP subunits highlighted in a close‐up view of the axial pore. Three out of five of these regions are in the up conformation (cyan) and two in the down conformation (dark blue). The denomination “up” refers to the β‐hairpin loop. N‐terminal regions that adopt the up conformation form a well‐defined, upwards pointing β‐hairpin structure and have their N‐termini (see N2) facing downwards. The two subunits adopting the down conformation are disordered and have their N termini facing upwards (see M1, N2) with the residues E9 to D19 extending into the axial channel and contributing to pore closure.
