Figure 5.
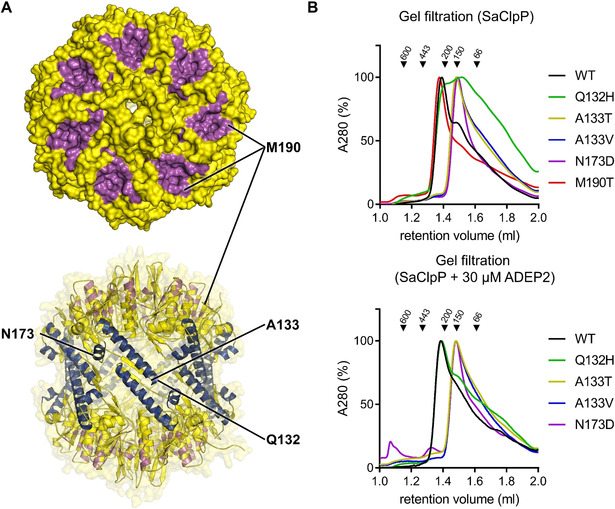
Analysis of the oligomeric state of mutated S. aureus 133 ClpP proteins selected under ADEP pressure. A) Positions of amino acid exchanges within ClpP pictured with the help of the apo‐SaClpP crystal structure (PDB ID: 6TTY) solved in this study. The top panel shows a top‐view surface representation of SaClpP with the ADEP binding sites coloured in magenta. The lower panel depicts a side‐view cartoon representation with the α5‐ and α6‐helices highlighted in dark blue. B) Gel filtration analysis of purified SaClpP mutant proteins. In the absence of ADEP, all but the M190T mutant show a defect in tetradecamer (301 kDa) formation. The Q132H mutant forms a mixture of different oligomeric states ranging from full tetradecamer to monomer (21.5 kDa) but can be transformed into a tetradecamer by the addition of ADEP. The mutants A133T, A133V, and N173D adopt a heptameric (150.5 kDa) conformation both in the absence and presence of ADEP. At an even higher ADEP concentration (60 μM), the mutants A133T, A133V and N173D showed a small proportion of tetradecamer (Figure S5). To assess the effect of ADEP, the proteins were preincubated with ADEP before being subjected to size‐exclusion chromatography, but the chromatography buffer contained no ADEP.
