Figure 4.
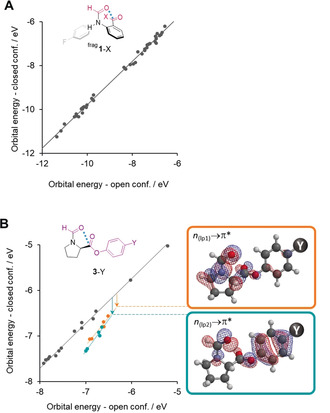
Correlation of calculated orbitals energies in the open vs. closed conformers of A) balance series 1‐X and B) Balance series 3‐Y. The second aromatic ring in balance series 1‐X was replaced with a proton to give frag 1‐X to avoid orbital splitting arising from the canonical resonance forms of the aromatic electrons (see Section S4.5). Data points that fall below the trend formed by grey points are stabilised in the closed conformer due to the n→π* electron delocalisation from both lone pairs of the carbonyl donor. For balance series frag 1‐X, no special stabilisation of orbitals between the open and closed conformations was observed, while for balance series 3‐Y two sets of molecular orbitals (orange and teal) were observed corresponding to stabilisation of the carbonyl oxygen lone pairs into the adjacent carbonyl group via n→π* interactions.
