Figure 5.
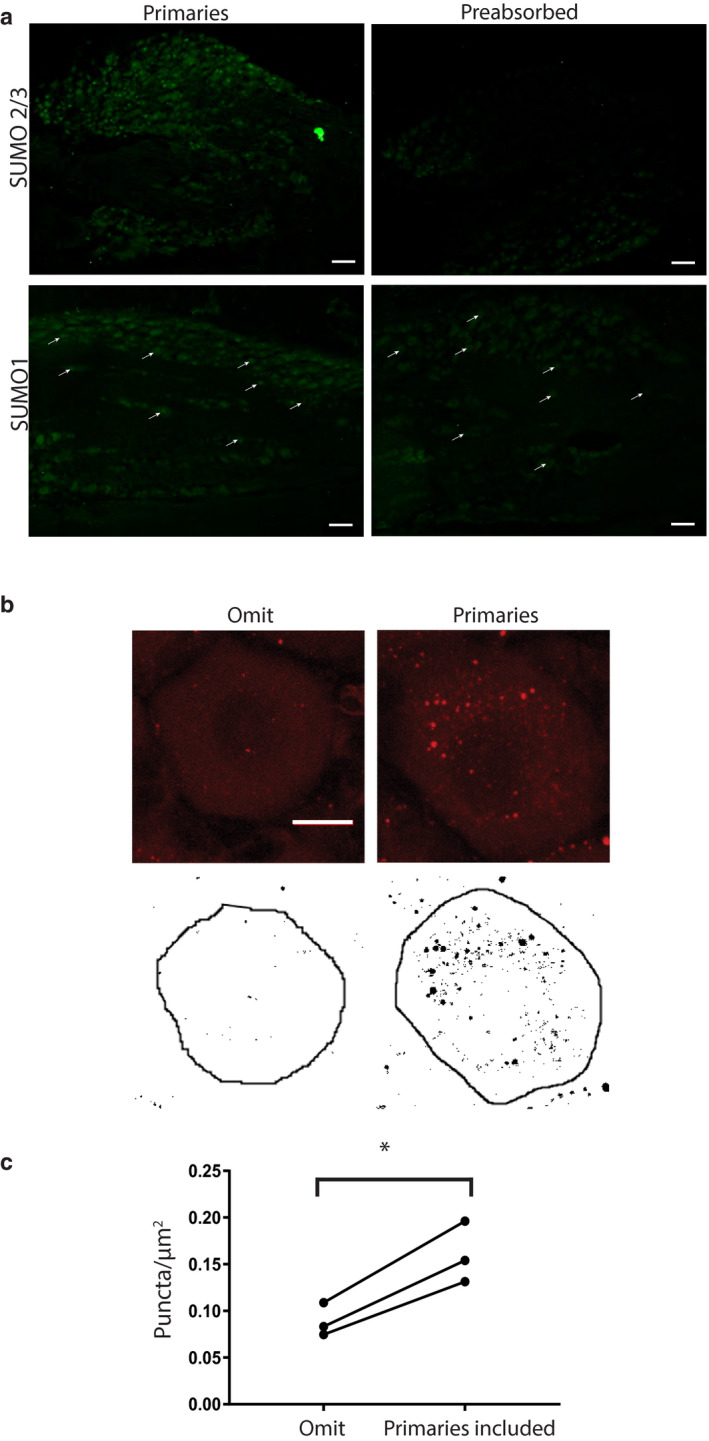
Measuring HCN2 channel SUMOylation. (a) Verification of SUMO antibodies. Antibodies were (right) or were not (left) preabsorbed with the corresponding peptide for SUMO2/3 (upper panel) or SUMO1 (lower panel). SUMO is predominately expressed in nuclei. Note the loss of nuclear staining following preabsorption, for example, white arrows in bottom panel. Scale bars are 100 µm. (b) Method for quantifying HCN2 channel SUMOylation. In situ PLA was performed on DRG cryosections with (experimental) or without (control) antibodies against SUMO2/3 and HCN2. Upper panel shows a 5 µm projection of confocal optical slices through representative cells from control and experimental treatment groups. Each image represents a projection of five slices in continuous succession that together encompass the centre of the cell. Each optical section is 0.9 µm with a z‐stack interval of 1 µm. Note that the red puncta indicating SUMOylated HCN2 channels were largely absent when antibodies were omitted. Scale bar is 10 µm. The cells were circle and thresholded, and the resulting image is shown in the lower panel. Black puncta within the circled region were counted using the analyse particle tool on imageJ and normalized by the area (µm2) of the circle. Note that all black puncta, regardless of size were counted. (c) HCN2 channels are SUMOylated in DRG neurons. Plots of puncta per µm2 show there are significantly more puncta when primary antibodies for HCN2 and SUMO2/3 were included (0.08 ± 0.01 vs. 0.16 ± 0.02, p = 0.0149, paired t‐test, n = 3, 70 and 62 cells analysed in total) *, p < 0.05. The lines indicate that the cells were from the same experiment, that is, alternate sections from a single DRG on the same slide receiving the same PLA reagents and treated in an identical fashion. DRG, dorsal root ganglia; HCN2, hyperpolarization‐activated, cyclic nucleotide‐gated 2; PLA, proximity ligation assays; SUMO, small ubiquitin like modifier
