Figure 3.
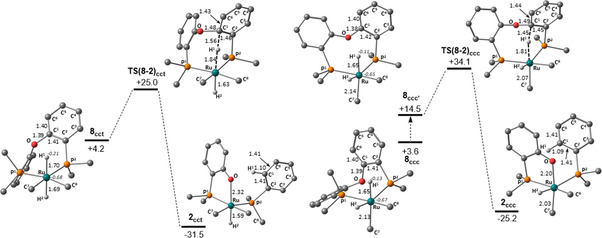
Computed free energy profiles (kcal mol−1, BP86(benzene, D3BJ)) for hydride attack in 8cct and 8ccc, with selected distances in Å. Energies are relative to 1 plus free DPEphos and NBO charges at Ru and H1 are indicated in italics for dihydride precursors. For clarity, IMe4 ligands are truncated at the C2 position (i.e. C7 and C8 in the Figure) and phenyl substituents at the ipso carbons. DPEphos hydrogens are also omitted. 8ccc’ is a conformer of 8ccc that lies directly on the pathway for C−O cleavage (see text for details).
