FIGURE 7.
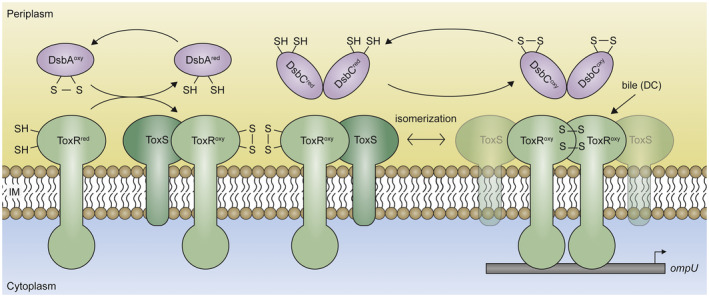
Activation mode and interaction patterns of ToxRS. Integral membrane proteins such as ToxRS are inserted into the cytoplasmic membrane co‐translationally, whereby ToxR exposes its reduced cysteine residues to the periplasm. These cysteines are then oxidized by DsbA to form intramolecular disulfide bonds, which represent the proteolytically stable form of ToxR. Increased ToxR‐ToxR PPIs can be detected if ToxS and ToxR‐boxes (e.g., ompU promoter) are available. Such an interaction is further strengthened in the presence of bile (sodium deoxycholate). As a model, we suggest that while ToxR molecules are bound to their operator sequences (ToxR‐boxes), temporarily formed ToxR dimers may exist due to intermolecular disulfide bond formation depending on DsbC or other mechanisms [Colour figure can be viewed at wileyonlinelibrary.com]
