Abstract
Background
There are sparse real‐world data on severe asthma exacerbations (SAE) in children. This multinational cohort study assessed the incidence of and risk factors for SAE and the incidence of asthma‐related rehospitalization in children with asthma.
Methods
Asthma patients 5‐17 years old with ≥1 year of follow‐up were identified in six European electronic databases from the Netherlands, Italy, the UK, Denmark and Spain in 2008‐2013. Asthma was defined as ≥1 asthma‐specific disease code within 3 months of prescriptions/dispensing of asthma medication. Severe asthma was defined as high‐dosed inhaled corticosteroids plus a second controller. SAE was defined by systemic corticosteroids, emergency department visit and/or hospitalization all for reason of asthma. Risk factors for SAE were estimated by Poisson regression analyses.
Results
The cohort consisted of 212 060 paediatric asthma patients contributing to 678 625 patient‐years (PY). SAE rates ranged between 17 and 198/1000 PY and were higher in severe asthma and highest in severe asthma patients with a history of exacerbations. Prior SAE (incidence rate ratio 3‐45) and younger age increased the SAE risk in all countries, whereas obesity, atopy and GERD were a risk factor in some but not all countries. Rehospitalization rates were up to 79% within 1 year.
Conclusions
In a real‐world setting, SAE rates were highest in children with severe asthma with a history of exacerbations. Many severe asthma patients were rehospitalized within 1 year. Asthma management focusing on prevention of SAE is important to reduce the burden of asthma.
Keywords: asthma: epidemiology, asthma: risk factors, epidemiology: prevalence
Short abstract

Key Message.
There are few studies on the real‐life incidence of severe asthma exacerbations in children and the few available strongly vary in study design. In this large international retrospective cohort study we investigated the incidence of and risk factors for severe asthma exacerbations in 6 European databases using one single protocol and harmonized methods for data extraction and data analysis. We observed exacerbation rates ranging between 17 and 198/1000 person‐years in the different databases with high rehospitalization rates. This underscores the importance of enhanced patient care in children with asthma to achieve the ultimate goal of preventing asthma exacerbations.
1. INTRODUCTION
Asthma is an important public health problem and is responsible for a high number of missed school and work days; moreover, it can be associated with significant limitations on physical, social and professional/student aspects of life when it is not controlled.1, 2 Asthma exacerbations represent a decline in lung function with worsening of respiratory symptoms such as shortness of breath, cough and/or chest tightness.2 According to a joint statement of the European Respiratory Society and the American Thoracic Society, a severe asthma exacerbation (SAE) is defined as the use of systemic corticosteroids, emergency department (ED) visit or hospitalization all for reason of asthma.3
There are scarce international data on the incidence of SAE in asthma children in the general population. The Asthma Insights and Reality in Europe (AIRE) study was conducted in 1999, in which asthma patients from 7 different European countries were questioned during a telephone interview. It showed differences in SAE rates between countries, as 7%‐40% of children with asthma reported an ED visit for asthma and 3%‐12% were hospitalized in the previous year.4 As this study used interview data, it was susceptible to recall bias. Randomized controlled trials can also provide data on the incidence of severe asthma exacerbations, but these numbers tend to be much lower than in real life due to strict inclusion and exclusion criteria and short follow‐up.5 Risk factors for SAEs have been studied primarily in adults, and thus, data in children are scarce.
Currently, we still lack reliable instruments or biomarkers to predict SAE in children. Several studies found that the best predictor of future SAE was a history of a previous SAE.6, 7 This implies that the risk of rehospitalization is probably high.
In this study, we aimed to estimate real‐world incidence rates of and risk factors for SAEs, as well as to study the rate and cumulative incidence of asthma rehospitalization in children with asthma and severe asthma, using one protocol and harmonized methods with regard to data extraction and data analysis, across multiple data sources from five different European countries.
2. METHODS
A retrospective cohort study was conducted using data from six European electronic healthcare databases, namely the Integrated Primary Care Information Project (IPCI) from the Netherlands, the Health Search Database (HSD) and Pedianet from Italy, Clinical Practice Research Datalink (CPRD) from the UK, the Sistema d'Informació per al Desenvolupament de la Investigació en Atenció Primària (SIDIAP) from Spain and the Aarhus University Prescription Database (Aarhus) from Denmark. Detailed descriptions of these databases have been published before and are available in the Appendix S1.8, 9, 10, 11, 12, 13 All databases comprise detailed information on drug prescriptions or dispensing, outpatient diagnoses and hospitalizations, comorbidity and measurement data (eg laboratory results, spirometry, BMI). While in most databases children of all ages are captured, in Italy for children 0‐6 years old all primary care is provided by paediatricians, and parents can choose between a paediatrician and a GP for children 6‐16 years of age. Therefore, Pedianet (primary care paediatrician data) tends to contain younger children, while HSD (GP data) tends to capture older children.
All participating databases comply with EU guidelines on the use of medical data for research and are registered in the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) database.14 In all 5 countries, the study protocol was approved by their local institutional review board.
2.1. Cohort definition
A cohort of patients with asthma was determined in each database within the study period (1 January 2008‐31 December 2013). To enter the cohort, patients needed to be 5‐17 years old, with a minimum of 1‐year database history and diagnosed with asthma. Asthma was defined as physician‐diagnosed asthma based on the presence of at least one asthma‐specific disease code (see Appendix S1) in combination with prescriptions/dispensing of asthma medications within 3 months of the asthma disease code. Asthma medications consisted of the following: inhaled corticosteroids (ICS), short‐acting β2 agonists (SABA), long‐acting β2 agonists (LABA), fixed combination of ICS + LABA, leukotriene receptor antagonist, short‐acting muscarinic antagonist (SAMA), fixed combination of SABA + SAMA, xanthines, systemic corticosteroids for the treatment of asthma, and anti‐IgE treatment. Information on drug use was retrieved by an ATC‐specific search from the drug prescription and/or drug dispensing records. Based on the first date of an asthma disease code, patients were categorized into prevalent or incident asthma.
Within the cohort of patients with asthma, a subcohort of patients with severe asthma was nested. According to international guidelines, severe asthma was defined as asthma requiring treatment with high‐dose ICS (as defined in the Global Initiative of Asthma (GINA); see Appendix S1) plus a second controller and/or systemic corticosteroids for at least 120 consecutive days.2, 15 To account for immortal time bias, the start date of severe asthma was day 120 of high‐dose ICS use.
2.2. Follow‐up
Follow‐up started at the latest date of the following: the start of the study period, diagnosis of (severe) asthma, age of 5 years or after reaching 365 days of database history. Follow‐up ended when leaving the database, reaching the age of 18, death or end of the study period, whichever came first.
2.3. Outcomes
An SAE was defined as any of the following: use of systemic corticosteroids, ED visit or hospitalization, all for worsening of asthma. 3 The indication of corticosteroid use was retrieved from the prescription/dispensing file or through an automated search on asthma (exacerbation) disease codes in a 7‐day window around the prescription date. Continuous use of systemic corticosteroids, defined as consecutive use of at least 30 days, was not considered as an SAE. If the time between exacerbations, of whatever type, was <7 days, it was considered to be one single exacerbation.
Rehospitalization was defined as an asthma‐related hospitalization following a previous asthma‐related hospitalization. Hospitalizations were based on primary discharge diagnosis codes in SIDIAP, Aarhus and CPRD and were based on manual validation of discharge letters in the other databases.
2.4. Covariates
We investigated the prevalence of the following comorbidities at the start of follow‐up and at each exacerbation, using the complete medical history: atopy (allergic rhinitis and/or atopic eczema/dermatitis), chronic rhinosinusitis, nasal polyposis, gastro‐oesophageal reflux disease (GERD), obesity and diabetes mellitus. In addition, we investigated whether or not patients had a history of exacerbations. History of exacerbations was defined as ≥2 SAEs in the 1 year prior to entering the (severe) asthma cohort. If patients had <12 months prior to asthma diagnosis or the start of severe asthma, 1 exacerbation or more in the 6 months prior fulfilled the criterion. We also investigated age and gender as covariates.
2.5. Analysis
The exacerbation rates were calculated by dividing the number of exacerbations by the respective number of person‐years of follow‐up and additionally stratified by gender and age in years.
Asthma rehospitalization rates in predefined follow‐up time windows (30, 180 and 365 days following a previous hospitalization) were calculated. If a patient had more than one exacerbation during that time window, each exacerbation contributed to the nominator. Furthermore, from these rates cumulative incidences were calculated.
Heterogeneity was quantified by the I2 statistics to assess if it was appropriate to perform a meta‐analysis on the overall SAE rates estimated in the databases.
Risk factors for exacerbations were assessed by Poisson regression analyses, adjusting for age, gender and their interaction. A multivariate model was fitted using the following covariates: age, gender, comorbidity (atopy, obesity, GERD), history of exacerbations and the interaction between age and gender and between age and history of exacerbations. These analyses were repeated after stratification for age: for children 5‐11 years old and for children 12‐17 years old. Analyses were conducted using SAS® version 9.4.
3. RESULTS
The cohort comprised 212 060 children with asthma contributing 678 625 patient‐years (PY). In total, 14 283 children (6.7%, ranging from 1.6% to 18.3% across databases) with severe asthma were identified.
The mean age at the start of follow‐up was 10.4 years (ranging between 7.2 and 14.8 years across databases), and a male preponderance was observed. The prevalence of atopy was 9.6% in Aarhus and ranged between 24.1% and 43.6% in the other databases. In all databases, the prevalence of atopy, GERD and obesity was higher in patients with severe asthma. (Tables 1 and 2).
Table 1.
Baseline characteristics of total asthma cohorts
| CPRD (n, %) | SIDIAP (n, %) | IPCI (n, %) | AARHUS (n, %) | PEDIANET (n, %) | HSD (n, %) | |
|---|---|---|---|---|---|---|
| Total | 124 554 | 30 749 | 31 705 | 12 521 | 8264 | 4267 |
| Female | 51 667 (41.5) | 12 357 (40.2) | 13 262 (41.8) | 4681 (37.4) | 2980 (36.1) | 1665 (39.0) |
| Male | 72 887 (58.5) | 18 392 (59.8) | 18 443 (58.2) | 7840 (62.6) | 5284 (63.9) | 2602 (61.0) |
| Age (mean, min‐max) | 10.9 (5.0‐17.9) | 8.9 (5.0‐17.9) | 10.2 (5.0‐17.9) | 9.3 (5.0‐17.9) | 7.2 (5.0‐14.0) | 14.8 (6.6‐17.9) |
| Severe asthma | 9162 (7.4) | 1139 (3.7) | 1858 (5.9) | 1207 (9.6) | 137 (1.7) | 780 (18.3) |
| Incident asthma | 23 743 (19.1) | 13 062 (42.5) | 4604 (14.5) | 1392 (11.1) | 1892 (22.9) | 1302 (30.5) |
| Prevalent asthma | 100 811 (80.9) | 17 687 (57.5) | 27 101 (85.5) | 11 129 (88.9) | 6372 (77.1) | 2965 (69.5) |
| History of exacerbations | ||||||
| Yes | 5276 (4.2) | 2625 (8.5) | 241 (0.8) | 408 (3.3) | 196 (2.4) | 321 (7.5) |
| No | 119 278 (95.7) | 28 124 (91.5) | 31 464 (99.2) | 12 113 (96.7) | 8068 (97.6) | 3946 (92.5) |
| Atopy | 54 356 (43.6) | 9767 (31.8) | 11 602 (36.6) | 1200 (9.6) | 1990 (24.1) | 961 (22.5) |
| GERD | 3247 (2.6) | 248 (0.8) | 510 (1.6) | 103 (0.8) | 146 (1.8) | 55 (1.3) |
| Diabetes mellitus | 294 (0.2) | 42 (0.1) | 81 (0.3) | 43 (0.3) | 0 | 7 (0.2) |
| Obesity | 24 742 (19.9) | 12 828 (41.7) | 1518 (5.0) | 205 (1.6) | 3977 (48.1) | 265 (6.2) |
| Chronic rhinosinusitis | 4917 (3.9) | 33 (0.1) | 112 (0.4) | 0 | 0 | 38 (0.9) |
| Nasal polyposis | 228 (0.2) | 0 | 13 (0.0) | 8 (0.1) | 0 | 0 |
| Patients with ≥1 exacerbation | 12 638 (10.2) | 8968 (29.3) | 871 (2.8) | 756 (6.0) | 724 (8.8) | 855 (20.0) |
| Asthma exacerbations (n) | 19 530 | 18 808 | 1290 | 1020 | 1031 | 1461 |
| Systemic corticosteroids (n, %) | 15 049 (77.0) | 17 323 (92.1) | 739 (57.3) | 252 (24.7) | 918 (89.0) | 1460 (99.9) |
| ED/ hospitalization (n, %) | 4481 (23.0) | 1485 (7.9) | 551 (42.7) | 768 (75.3) | 113 (11.0) | 1 (0.1) |
Abbreviations: ED, emergency department; GERD, gastro‐oesophageal reflux disease.
Table 2.
Baseline characteristics of severe asthma cohorts
| CPRD (n, %) | SIDIAP (n, %) | IPCI (n, %) | AARHUS (n, %) | PEDIANET (n, %) | HSD (n, %) | |
|---|---|---|---|---|---|---|
| Total | 9162 | 1139 | 1858 | 1207 | 137 | 780 |
| Female | 3705 (40.4) | 481 (42.2) | 806 (43.4) | 406 (33.6) | 34 (24.8) | 292 (37.4) |
| Male | 5457 (59.6) | 658 (57.8) | 1052 (56.6) | 801 (66.4) | 103 (75.2) | 488 (62.6) |
| Age (mean, min‐max) | 11.0 (5.0‐17.9) | 8.60 (5.0‐17.9) | 13.1 (5.0‐17.9) | 10.4 (5.0‐17.9) | 9.3 (5.0‐12.9) | 14.5 (2.4) |
| Incident asthma | 436 (4.8) | 164 (14.4) | 122 (6.6) | 102 (8.5) | 6 (4.4) | 79 (10.1) |
| Prevalent asthma | 8726 (95.2) | 975 (85.6) | 1736 (93.4) | 1105 (91.5) | 131 (95.6) | 701 (89.9) |
| History of exacerbations | ||||||
| Yes | 562 (6.1) | 215 (18.9) | 49 (2.6) | 40 (1.7) | 1 (0.8) | 55 (7.1) |
| No | 8600 (93.9) | 924 (81.1) | 1809 (97.4) | 1167 (98.3) | 136 (99.3) | 725 (92.9) |
| Atopy | 4724 (51.6) | 407 (35.7) | 789 (42.5) | 194 (16.1) | 54 (39.4) | 190 (24.4) |
| GERD | 375 (4.1) | 23 (2.0) | 34 (1.8) | 14 (1.2) | 3 (2.2) | 16 (2.1) |
| Diabetes mellitus | 25 (0.3) | 2 (0.2) | 4 (0.2) | 6(0.5) | 0 | 2 (0.3) |
| Obesity | 2541 (27.7) | 522 (45.8) | 174 (9.4) | 45 (3.7) | 60 (43.8) | 57 (7.3) |
| Chronic rhinosinusitis | 475 (5.2%) | 3 (0.3%) | 12 (0.6%) | 0 | 0 | 7 (0.9%) |
| Nasal polyposis | 31 (0.3%) | 0 | 3 (0.2%) | 1 (0.1%) | 0 | 0 |
Abbreviation: GERD, gastro‐oesophageal reflux disease
Of all asthma patients, depending on the database, 2.8%‐29.3% had at least one exacerbation during the follow‐up. In HSD, SIDIAP and PEDIANET, SAE mostly comprised use of systemic corticosteroids (89%‐100%), while in IPCI, Aarhus and CPRD, this proportion was 57%, 25% and 77%, respectively. Large differences between databases in incidence rates (IR) of SAE were observed especially for the younger age. The overall SAE rates ranged between 17 and 198/1000 PY and were higher in severe asthma patients (range 46‐375/1000 PY). SAE rates decreased with age, from age 5 onwards, and this was significant for all databases. (Figure 1 and Table 3) From adolescence on, higher IRs of SAE were observed for women compared to men in CPRD, SIDIAP, IPCI and Aarhus. (Figure 1) The IR ranged between 11 and 131/1000 PY in patients without a history of exacerbations and was higher in children with severe asthma especially with a history of exacerbations (283‐1000/1000 PY). (Figure 2 and Table S1) As the heterogeneity of exacerbation rates was substantial (I 2 > 99%), it was not appropriate to pool the data.
Figure 1.
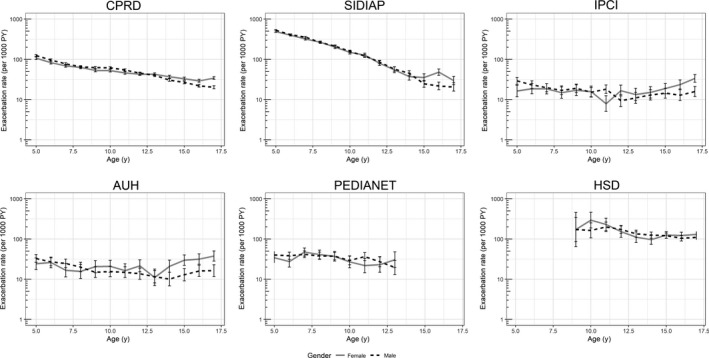
SAE rate (per 1000 PY) with 95% CI by age and gender in the total asthma cohort per database
Table 3.
Relative rates for SAE in the total asthma cohort (multivariate analysis)
| Characteristic | CPRD | SIDIAP | IPCI | AARHUS | PEDIANET | HSD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | |
| Age | 0.89 | (0.88, 0.89) | 0.78 | (0.78, 0.79) | 0.94 | (0.92, 0.96) | 0.95 | (0.92,0.97) | 0.94 | (0.91, 0.97) | 0.95 | (0.91, 0.99) |
| Female | 0.68 | (0.62, 0.74) | 0.83 | (0.76, 0.91) | 0.43 | (0.30, 0.61) | 0.39 | (0.26,0.57) | 0.88 | (0.55, 1.41) | 0.46 | (0.21, 1.02) |
| Age*Sex | 1.04 | (1.03, 1.05) | 1.02 | (1.01, 1.03) | 1.09 | (1.06, 1.12) | 1.11 | (1.07,1.15) | 1.01 | (0.95, 1.06) | 1.06 | (1.00, 1.11) |
| Atopy | 1.37 | (1.34, 1.41) | 1.09 | (1.06, 1.12) | 1.51 | (1.36, 1.69) | 2.07 | (1.75,2.44) | 1.17 | (1.01, 1.34) | 0.92 | (0.81, 1.05) |
| GERD | 1.18 | (1.09, 1.28) | 1.48 | (1.30, 1.67) | 1.53 | (1.08, 2.17) | 1.15 | (0.57,2.31) | 1 | (0.62, 1.61) | 1.1 | (0.67, 1.80) |
| Obesity | 1.25 | (1.21, 1.29) | 0.9 | (0.88, 0.93) | 1.19 | (0.94,1.51) | 2.14 | (1.55,2.96) | 1.02 | (0.90, 1.15) | 0.92 | (0.73 1.16) |
| History of exacerbations | 5.76 | (5.25, 6.33) | 2.53 | (2.27, 2.81) | 20.04 | (12.91, 31.10) | 45.71 | (31.2, 66.92) | 29.36 | (16.25, 53.05) | 10.07 | (4.56, 22.20) |
| Age*History of exacerbations | 1.06 | (1.05, 1.07) | 1.01 | (1.00, 1.03) | 1.04 | (1.00,1.08) | 0.94 | (0.91,0.97) | 0.91 | (0.85, 0.98) | 0.97 | (0.92, 1.02) |
Abbreviation: GERD, gastro‐oesophageal reflux disease
Figure 2.
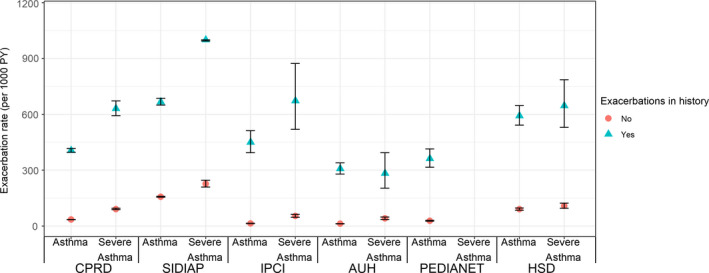
SAE rate (per 1000 PY) with 95% CI by history of exacerbations and severity (severe asthma in Pedianet not presented as low numbers)
3.1. Risk factors for severe asthma exacerbations
After adjustment for age, gender and their interaction, having atopy, GERD and obesity increased the risk of SAE in some databases. (Table S2) In the multivariate model, the same risk factors remained, but risk estimates were lower. (Table 3) Additionally, the interaction between age and a history of exacerbations showed that in some databases, the risk associated with a history of exacerbations increased with increasing age, and in others, this risk decreased. After stratification for age category, for female gender in 5‐ to 11‐year‐olds a decreased risk was found, while in 12‐ to 17‐year‐olds there was an increased risk. These findings were less prominent in Pedianet and HSD, possibly due to the limited age range in these databases. No other distinct differences between the age categories were observed (see Appendix S1).
3.2. Rehospitalization following previous hospitalization for asthma exacerbation
Rehospitalization rates decreased with time following index hospitalization. The asthma rehospitalization rate was highest in SIDIAP (within 1 month: 13.7/10 PY in children with asthma and 88.3/10 PY in children with severe asthma), whereas in the other databases the rehospitalization rate within the first month ranged between 5.4 and 7.7/10 PY in children with asthma and between 14.3 and 15.8/10 PY in children with severe asthma.
The cumulative incidence of rehospitalization within 1 year was 17%‐29% in children with asthma, whereas this was much higher, namely 33%‐79%, in patients with severe asthma. (Table 4) No data are presented for HSD and Pedianet as the number of children being hospitalized for asthma exacerbation was too low.
Table 4.
Asthma rehospitalization per 10 person‐years following discharge from previous asthma hospitalization in different time periods per database (HSD and PEDIANET not presented as low numbers)
| <30 d after hospitalization | <180 d after hospitalization | <365 d after hospitalization | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rehospitalizations (n) | Rate/10 PY (95% CI) | Cumulative incidence (%) | Rehospitalizations (n) | Rate/10 PY (95% CI) | Cumulative incidence (%) | Rehospitalizations (n) | Rate/10 PY (95% CI) | Cumulative incidence (%) | |
| CPRD | |||||||||
| Asthma | 121 | 5.4 (4.5,6.5) | 4.3 | 257 | 2.1 (1.9,2.4) | 9.8 | 421 | 1.9 (1.7,2.1) | 17.3 |
| Severe asthma | 58 | 15.7 (12.2, 20.4) | 12.1 | 100 | 5.3 (4.4,6.4) | 23.0 | 137 | 4.0 (3.4,4.8) | 33.0 |
| SIDIAP | |||||||||
| Asthma | 87 | 13.7 (11.1, 16.9) | 10.6 | 164 | 5.1 (4.3,5.9) | 22.2 | 205 | 3.4 (3.0,3.9) | 28.8 |
| Severe asthma | 58 | 88.3 (68.3, 114.2) | 51.6 | 62 | 25.1 (19.5, 32.2) | 71.0 | 67 | 15.8 (12.4, 20.0) | 79.4 |
| IPCI | |||||||||
| Asthma | 12 | 6.4 (3.6, 11.2) | 5.1 | 37 | 3.9 (2.9,5.4) | 17.5 | 48 | 3.0 (2.2,3.9) | 25.9 |
| Severe asthma | 6 | 14.3 (6.4, 31.8) | 11.1 | 20 | 10.8 (7.0, 16.8) | 41.3 | 22 | 7.3 (4.8, 11.1) | 51.8 |
| AARHUS | |||||||||
| Asthma | 44 | 7.7 (5.7, 10.3) | 6.1 | 98 | 3.1 (2.6,3.8) | 14.2 | 133 | 2.3 (1.9,2.7) | 20.0 |
| Severe asthma | 16 | 15.8 (9.7, 25.7) | 12.2 | 32 | 6.3 (4.4,8.9) | 26.7 | 46 | 5.1 (3.8,6.8) | 40.0 |
Abbreviations: 95% CI, 95% confidence interval; PY, person‐years.
4. DISCUSSION
In this study, the overall exacerbation rate in children with asthma ranged between 17 and 198/1000 PY across databases, increased in children with severe asthma (range 46‐375/1000 PY) and was the highest in those with severe asthma with a history of exacerbations (283‐1000/1000 PY). Rehospitalization following a previous hospitalization was high, both in the overall asthma cohort, namely 17%‐29% being rehospitalized in a year, and especially in children with severe asthma, as up to 79% were rehospitalized within a year.
We observed large differences in incidence rate of SAE, especially for the youngest age categories, and also large differences in the type of SAE. Large differences in exacerbation rates between European countries have previously been observed in other studies that used questionnaires or interviews to collect data instead of healthcare databases. In line with our results, these studies showed higher severe exacerbation rates in Italy and Spain than in the UK, and higher rates of emergency care in Spain than in the UK. In the Asthma Insights and Reality in Europe (AIR) survey, hospitalization rates in children were higher in Spain and Italy than in The Netherlands and the UK. 16, 17, 18 In these studies, many reasons for these differences were suggested: differences in disease management such as asthma prevention plans, lung function measurement, and difference in drug prescriptions, compliance with therapy, environmental differences and cultural differences such as perception of disease burden and healthcare‐seeking behaviour.
Our exacerbation rates are in line with a recent study by Suruki et al investigating SAE in adolescent and adult asthma patients.19 They reported a mean exacerbation rate in asthma patients 12‐17 years old of 156/1000 PY based on US claims data and of 77/1000 PY using CPRD data. Comparison with other studies is difficult, as most of these only investigated hospitalizations for asthma, included systemic corticosteroids irrespective of the indication of use or investigated a selective population.17, 19, 20, 21, 22, 23, 24
Our observation on frequent rehospitalization is in line with data from two cohort studies on hospitalizations for children with asthma in the United States which observed that 17% and 22% of hospitalized children were readmitted within 365 days.25, 26 The risk factors for SAE were a previous SAE, male gender, youngest age and atopy. In agreement with published literature, in our study a previous exacerbation was the best predictor of a subsequent asthma exacerbation.27, 28, 29 Other published risk factors for asthma exacerbations such as obesity and GERD could not be confirmed as risk factor in all participating databases.30, 31
As for all observational studies, our study has strengths and limitations. Major strengths include the large number of children with asthma included in our cohort, from 6 different European databases in 5 countries, using a single protocol and harmonized methods for data extraction and data analysis. Detailed information on important covariates such as drug exposure and underlying comorbidity and on lifestyle factors was collected. The use of real‐life data in a heterogeneous asthmatic population increases the external validity of our findings as our data are highly representative of actual practice conditions and likely to minimize Hawthorne and similar effects.32
However, as this is an observational study, using data from electronic healthcare databases, there is always the potential of bias and/or confounding.
First, we did report large differences in the incidence rates of SAE between countries.
For those databases where automatic linkage with hospital data was available (CPRD, SIDIAP and Aarhus), misclassification of ED visits/hospitalization is unlikely. For the other databases, hospitalization or ED visit was retrieved either via disease‐specific codes in combination with codes for hospitalization (HSD and Pedianet) or via review of the discharge letters (IPCI). For prescriptions of systemic steroids, only those with an indication for asthma registered in the database were included. If practices did not report the indication of use or did not report disease codes around the prescription date, we might have underreported the incidence rate. Similar underreporting might have occurred if patients used previously collected systemic corticosteroids.
Also, the large differences in SAE rates might be due to international differences in the use of systemic steroids. For example, in Spain (SIDIAP), a large incidence of SAE based on use of systemic steroids was observed in young children. Despite the fact that these differences between the databases complicate comparison between countries, we believe that it is better to study six different databases in one identical way than having six different single database studies using different methods because that would make comparison even more difficult.
For Aarhus and SIDIAP, dispensing data instead of prescription data were used, reducing misclassification of exposure. Unfortunately, dosing information is missing in Aarhus and SIDIAP and dosing was derived from the strength per device and the time window between prescriptions. Of course, this method is prone to misclassification, which might explain the relative low proportion of severe asthma patients in SIDIAP.
Because of large numbers, manual validation of asthma was not feasible and asthma was defined based on the presence of disease codes, and thus, potential misclassification of asthma cannot be excluded. However, to ensure good quality of health care, GPs are trained and requested to enter disease codes fitting the actual patient's diagnosis.
Furthermore, for each of the comorbidities of interest, diseases were mapped through the Unified Medical Language System (UMLS) generating a list of disease codes (see Appendix S1) which were verified by the databases prior to extraction.33 Still we cannot exclude potential misclassification in case certain disease codes were omitted or in case of country‐specific differences in disease coding. Despite these potential limitations, we were able to give a good reflection of exacerbations in the general population and found similar trends of risk factors across the databases.
5. CONCLUSION
In this study, the incidence rates of severe asthma exacerbation in children ranged between 17 and 198/1000 PY and increased in children with severe asthma. In keeping with previous studies, the most important risk factor for hospitalization was a previous exacerbation.
Rehospitalization for asthma following a previous hospitalization was high, especially in children with severe asthma as up to 50% were rehospitalized within 1 month and up to 79% within 1 year following discharge. This underscores the importance of enhanced patient care in children with asthma to achieve the ultimate goal of preventing asthma exacerbations.
CONFLICT OF INTEREST
FA, SC, EB, LAE and MKVD are employees of GSK and own stocks/shares in GSK. NB and RS were employees of GSK at the time this research was conducted and own stocks/shares in GSK. EJB, GP, CG and KB have no conflicts to declare. FL has received grants from Chiesi, GSK and Novartis. DPA has received research grants from Amgen, Bioiberica and GSK and speaker/advisory fees from Amgen and Bioiberica, paid to his department. ME has received grants from GSK and ZonMw. The institutions of MDR, PR, MS and KV have received unconditional research grants from Boehringer‐Ingelheim, Novartis, Pfizer, Yamanouchi, Servier and Johnson & Johnson, unrelated to the current manuscript; MDR, MS and KV have received an unconditional grant from GSK to conduct research on the incidence of and risk factors for asthma exacerbations as part of the GSK/EU‐ADR Alliance project. HMJ has received grants from NIH and NCFS, participates in studies funded by an unconditional research grant from Vectura, and received travel expenses from Vertex, all unrelated to the current manuscript. ES’s institution (Aarhus University) has received a grant from the GSK/EU‐ADR Alliance, related to this study.
AUTHOR CONTRIBUTIONS
ME, EJB, MDR, ES, DPA, FL, GP, RS, PR, MS, NB and KV contributed to the study concept and design and acquisition of data and were involved in data analysis and interpretation. FA, EB, LAE, CG, SC, MKVD and EJB contributed to the study concept and design and were involved in data analysis and interpretation. All authors were involved in the preparation and review of the manuscript and approved the submitted version.
Supporting information
AppendixS1
ACKNOWLEDGMENTS
Funding for this study was provided by GSK (PRJ2284) and the ZonMw (ME and KV) (113201006). All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. This work was carried out through the EU‐ADR Alliance. The authors wish to acknowledge Eva Molero and Natasha Yefimenko (Synapse Research Management Partners) for their contributions to managing the study protocol during the development of this manuscript.
Engelkes M, Baan EJ, de Ridder MAJ, et al. Incidence, risk factors and re‐exacerbation rate of severe asthma exacerbations in a multinational, multidatabase pediatric cohort study. Pediatr Allergy Immunol. 2020;31:496–505. 10.1111/pai.13237
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13237
Funding information
GSK (PRJ2284) and ZonMw (113201006).
REFERENCES
- 1. Blakey JD, Wardlaw AJ. What is severe asthma? Clin Exp Allergy. 2012;42(5):617‐624. [DOI] [PubMed] [Google Scholar]
- 2. GINA . Pocket guide for asthma management and prevention. 2019.
- 3. Reddel HK, Taylor DR, Bateman ED, et al. An official American thoracic society/European respiratory society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59‐99. [DOI] [PubMed] [Google Scholar]
- 4. Vermeire PA, Rabe KF, Soriano JB, Maier WC. Asthma control and differences in management practices across seven European countries. Respir Med. 2002;96(3):142‐149. [DOI] [PubMed] [Google Scholar]
- 5. Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128(6):1165‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haselkorn T, Fish JE, Zeiger RS, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the expert panel report 3 guidelines, increases risk for future severe asthma exacerbations in the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) study. J Allergy Clin Immunol. 2009;124(5):895‐902.e4. [DOI] [PubMed] [Google Scholar]
- 7. Miller MK, Lee JH, Miller DP, Wenzel SE, Group TS . Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101(3):481‐489. [DOI] [PubMed] [Google Scholar]
- 8. Vlug AE, van der Lei J, Mosseveld BM, et al. Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med. 1999;38(4‐5):339‐344. [PubMed] [Google Scholar]
- 9. Ehrenstein V, Antonsen S, Pedersen L. Existing data sources for clinical epidemiology: Aarhus university prescription database. Clin Epidemiol. 2010;2:273‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cazzola M, Puxeddu E, Bettoncelli G, et al. The prevalence of asthma and COPD in Italy: a practice‐based study. Respir Med. 2011;105(3):386‐391. [DOI] [PubMed] [Google Scholar]
- 11. Cricelli C, Mazzaglia G, Samani F, et al. Prevalence estimates for chronic diseases in Italy: exploring the differences between self‐report and primary care databases. J Public Health Med. 2003;25(3):254‐257. [DOI] [PubMed] [Google Scholar]
- 12. Gulliford MC, van Staa T, Dregan A, et al. Electronic health records for intervention research: a cluster randomized trial to reduce antibiotic prescribing in primary care (eCRT study). Ann Fam Med. 2014;12(4):344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sorensen HT, Larsen BO. A population‐based Danish data resource with possible high validity in pharmacoepidemiological research. J Med Syst. 1994;18(1):33‐38. [DOI] [PubMed] [Google Scholar]
- 14. Blake KV, Devries CS, Arlett P, Kurz X, Fitt H. Increasing scientific standards, independence and transparency in post‐authorisation studies: the role of the European network of centres for pharmacoepidemiology and pharmacovigilance. Pharmacoepidemiol Drug Saf. 2012;21(7):690‐696. [DOI] [PubMed] [Google Scholar]
- 15. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343‐373. [DOI] [PubMed] [Google Scholar]
- 16. Vermeire PA, Rabe KF, Soriano JB, Maier WC. Asthma control and differences in management practices across seven European countries. Respir Med. 2002;96(3):142‐149. [DOI] [PubMed] [Google Scholar]
- 17. Henneberger PK, Mirabelli MC, Kogevinas M, et al. The occupational contribution to severe exacerbation of asthma. Eur Respir J. 2010;36(4):743‐750. [DOI] [PubMed] [Google Scholar]
- 18. Hyland ME, Sthl E. Asthma treatment needs: a comparison of patients' and health care professionals' perceptions. Clin Ther. 2004;26(12):2141‐2152. [DOI] [PubMed] [Google Scholar]
- 19. Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suruki RY, Boudiaf N, Ortega HG. Retrospective cohort analysis of healthcare claims in the United States characterising asthma exacerbations in paediatric patients. World Allergy Organ J. 2016;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bisgaard H, Moller H. Changes in risk of hospital readmission among asthmatic children in Denmark, 1978–93. BMJ. 1999;319(7204):229‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morrison DS, McLoone P. Changing patterns of hospital admission for asthma, 1981–97. Thorax. 2001;56(9):687‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kemp T, Pearce N. The decline in asthma hospitalisations in persons aged 0–34 years in New Zealand. Aust N Z J Med. 1997;27(5):578‐581. [DOI] [PubMed] [Google Scholar]
- 24. Mitchell EA, Cutler DR. Paediatric admissions to Auckland hospital for asthma from 1970–1980. N Z Med J. 1984;97(749):67‐70. [PubMed] [Google Scholar]
- 25. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300‐305. [DOI] [PubMed] [Google Scholar]
- 26. Auger KA, Kahn RS, Davis MM, Simmons JM. Pediatric asthma readmission: asthma knowledge is not enough? J Pediatr. 2015;166(1):101‐108. [DOI] [PubMed] [Google Scholar]
- 27. Price D, Wilson AM, Chisholm A, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 2016;9:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salamzadeh J, Wong IC, Hosker HS, Chrystyn H. A Cox regression analysis of covariates for asthma hospital readmissions. J Asthma. 2003;40(6):645‐652. [DOI] [PubMed] [Google Scholar]
- 29. Arabkhazaeli A, Vijverberg SJH, van Erp FC, Raaijmakers JAM, van der Ent CK, Maitland van der Zee AH. Characteristics and severity of asthma in children with and without atopic conditions: a cross‐sectional study. BMC Pediatr. 2015;15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Longo C, Bartlett G, Schuster T, Ducharme FM, MacGibbon B, Barnett TA. The obese‐asthma phenotype in children: an exacerbating situation? J Allergy Clin Immunol. 2018;141(4):1239‐1249.e4. [DOI] [PubMed] [Google Scholar]
- 31. Quinto KB, Zuraw BL, Poon KY, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol. 2011;128(5):964‐969. [DOI] [PubMed] [Google Scholar]
- 32. Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015;351:h4672. [DOI] [PubMed] [Google Scholar]
- 33. Gu H, Chen Y, He Z, Halper M, Chen L. Quality assurance of UMLS semantic type assignments using SNOMED CT hierarchies. Methods Inf Med. 2016;55(2):158‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1


